Abstract
Transthyretin (TTR) is a tetrameric protein synthesized primarily by the liver. TTR can misfold into pathogenic ATTR amyloid fibrils that deposit in the nerves and heart, causing a progressive and debilitating polyneuropathy (PN) and life-threatening cardiomyopathy (CM). Therapeutic strategies, which are aimed at reducing ongoing ATTR amyloid fibrillogenesis, include stabilization of the circulating TTR tetramer or reduction of TTR synthesis. Small interfering RNA (siRNA) or antisense oligonucleotide (ASO) drugs are highly effective at disrupting the complementary mRNA and inhibiting TTR synthesis. Since their development, patisiran (siRNA), vutrisiran (siRNA) and inotersen (ASO) have all been licensed for treatment of ATTR-PN, and early data suggest these drugs may have efficacy in treating ATTR-CM. An ongoing phase 3 clinical trial will evaluate the efficacy of eplontersen (ASO) in the treatment of both ATTR-PN and ATTR-CM, and a recent phase 1 trial demonstrated the safety of novel in vivo CRISPR-Cas9 gene-editing therapy in patients with ATTR amyloidosis. Recent results from trials of gene silencer and gene-editing therapies suggest these novel therapeutic agents have the potential to substantially alter the landscape of treatment for ATTR amyloidosis. Their success has already changed the perception of ATTR amyloidosis from a universally progressive and fatal disease to one that is treatable through availability of highly specific and effective disease-modifying therapies. However, important questions remain including long-term safety of these drugs, potential for off-target gene editing, and how best to monitor the cardiac response to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gene silencer and gene-editing therapies have completely transformed the landscape of treatment for ATTR amyloidosis. Directly targeting transthyretin production in patients with ATTR polyneuropathy has resulted in a tremendous improvement in outcomes. |
Small-scale studies that assessed the use of gene silencers in the treatment of ATTR cardiomyopathy have yielded promising results. With the results of large-scale trials expected shortly, gene silencers may also be approved for the treatment of ATTR cardiomyopathy in the near future. |
Despite the undeniable success of these disease-modifiers, important questions remain including long-term safety of these drugs, potential for off-target gene editing, and how best to monitor the cardiac response to treatment. |
1 Introduction
Systemic amyloidosis describes a heterogenous group of diseases characterised by amyloid fibril deposition within the extracellular space of various organs. Amyloid fibrils are formed when soluble precursor proteins misfold into insoluble, protease-resistant beta-pleated sheets that are histologically identified by apple-green birefringence when stained with Congo-Red dye and examined under cross-polarised light. Disease occurs when aggregation of amyloid fibrils within the extracellular matrix is sufficient to disrupt the structure, integrity and function of the affected organ [1, 2].
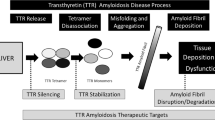
Transthyretin (TTR) amyloidosis, also known as ATTR amyloidosis, is caused by misfolding of the transthyretin protein. Transthyretin is a tetramer naturally synthesised by the liver, and a physiological transport protein for thyroxine and retinol. Disease occurs when transthyretin misfolds into pathogenic amyloid fibrils [3, 4]. In vitro formation of ATTR fibrils occurs when the tetrameric form of TTR becomes unstable and dissociates into monomers or is enzymatically cleaved. TTR instability appears to be promoted by oxidative modifications, age-related failure of homeostatic mechanisms, metal cations and inherited pathogenic genetic mutations [5].
Historically, ATTR amyloidosis was regarded as a rare disease, but advances in diagnostics, increased awareness, and the increasing perception of ATTR amyloidosis as a treatable disease have resulted in significantly increased diagnoses in recent years. Data from the National Amyloidosis Centre (the single UK centre for diagnosis and monitoring of amyloidosis) demonstrated an exponential increase in diagnoses over the last two decades. The incidence of ATTR amyloidosis increased from < 3% of all amyloidosis cases between 1987 and 2009, to 14% between 2010 and 2015, and 25% between 2011 and 2014 [6]. This trend has been observed in Sweden with the prevalence of ATTR amyloid cardiomyopathy (ATTR-CM) increasing from 1 per 100,000 in 2008, to five per 100,000 in 2018 [7]. Despite these improvements, ATTR amyloidosis still remains an underdiagnosed entity. The true prevalence remains unknown, but with autopsy studies demonstrating ATTR amyloid deposits in 25% of myocardial samples in patients aged ≥ 85 years, it is thought to be significantly higher than that reported in the literature [8].
ATTR amyloidosis may be either wild-type ATTR (ATTRwt), which occurs secondary to an acquired pathogenic process associated with aging, or hereditary ATTR (hATTR or ATTRv) amyloidosis, which occurs secondary to an inherited TTR-gene mutation. Each pathogenic TTR variant is caused by a single nucleotide substitution resulting in a missense mutation that is inherited in an autosomal dominant fashion with variable disease penetrance. The clinical phenotype depends on the underlying pathogenic TTR variant [9]. ATTRwt amyloidosis predominantly affects males and presents in later life [10]. Myocardial ATTR amyloid deposition results in a restrictive cardiomyopathy, characterised by biventricular wall thickening, stiffening of the myocardium, and development of systolic and diastolic dysfunction [11, 12]. Extra-cardiac sites of amyloid infiltration are associated with carpal tunnel syndrome, lumbar spine stenosis and tendinopathies [13,14,15]. ATTRv amyloidosis typically presents at a younger age with a mixed phenotype comprising polyneuropathy (ATTR-PN) and/or restrictive cardiomyopathy. ATTRv amyloid more commonly deposits in the nervous system than ATTRwt amyloid, and causes a progressive, length-dependent, sensory-motor peripheral polyneuropathy and/or autonomic neuropathy. Over 130 pathogenic TTR variants have been identified, but a small handful are responsible for the majority of cases [16]. Although there is considerable overlap, there is a strong association between the specific TTR variant, the clinical phenotype, and prognosis. However, phenotypic diversity does occur, not only between different causative TTR variants but also within variants.[17] For example p.(Val50Met), probably the most common disease-causing TTR variant worldwide, can manifest as early- or late-onset disease. Early-onset disease (age < 50 years) is associated with predominant ATTR-PN in the absence of cardiac amyloidosis (CA), while late-onset disease is associated with a mixed phenotype consisting of ATTR-PN and ATTR-CM, known as ATTR-Mixed [18]. In contrast, the p.(Val142Ile) variant, thought to be carried by up to 3–4% of African Americans, is strongly associated with a predominant cardiomyopathy [19]. Although ATTR-PN causes severe disabling symptoms, cardiac involvement is the main driver of mortality [20].
Early diagnosis and initiation of disease-modifying therapy is of paramount importance, and associated with reduced morbidity and mortality. Prior to the development of specific disease-modifying therapies, successful treatment of ATTRv-PN had been achieved through liver transplantation, which suppresses the production of variant TTR, and stops ATTRv fibril deposition in the nerves. Successful outcomes following liver transplantation supported the hypothesis that reducing or halting TTR production could prevent disease progression [5]. The last decade has seen multiple advances in the treatment of ATTR amyloidosis. Therapeutic strategies include targeted stabilisation of the transthyretin tetramer to prevent dissociation into amyloidogenic monomers or cleavage into amyloidogenic fragments [21, 22]. Recent years have also seen the development of agents aimed at reducing TTR production by disrupting the relevant messenger RNA (mRNA) with either small interfering RNA (siRNA) [23] or antisense oligonucleotides (ASO) [24], and an exciting novel approach that aims to halt TTR production through gene editing is being investigated [25]. While the majority of therapeutic strategies are aimed at slowing or preventing amyloid formation, it is noteworthy that monoclonal antibodies that target ATTR fibrils and accelerate their removal from vital organs are at various stages of development. If successful, these immunotherapeutic agents have the potential to facilitate organ recovery [5]. This review will explore in detail the current therapeutic strategies aimed at suppressing TTR production through gene silencing and gene editing, and also provide insights into future perspectives.
2 Small Interfering RNAs
siRNA are double-stranded RNA molecules that bind complementary mRNA molecules and cause degradation, hence interfering with gene expression. In order to be effective, siRNAs must be delivered into the cytoplasm of the target cells, while avoiding degradation from circulating nucleases and phagocytes, and renal filtration [26,27,28].
2.1 Patisiran
Patisiran was the first siRNA developed for ATTR amyloidosis, and was approved by the United States Food and Drug Administration (FDA) and European Commission (EC) in 2018 for the treatment of ATTRv-PN. Patisiran is formulated in a lipid nanoparticle to ensure delivery to the hepatocyte. Once released into the cytoplasm of the hepatocyte, the double-stranded siRNA splits into single-stranded RNAs that bind complementary mRNA. Binding triggers activation of the Argonaute slicer protein, which degrades the mRNA, thereby inhibiting TTR synthesis [29, 30]. In a phase 1 study of patisiran, single administration of 0.3 mg/kg achieved a maximum reduction in serum TTR concentration of 87.6% [31]. Mild/moderate renal impairment or mild hepatic impairment appeared to have no effect on the pharmacokinetics or TTR reduction [29], and patisiran has since been well tolerated in ATTRv-PN patients who underwent a previous liver transplant [32].
2.1.1 Neurological Outcomes
The APOLLO phase 3 trial was an 18 month randomised, placebo-controlled, double-blind study of 225 patients, which assessed the efficacy and safety of patisiran in ATTRv-PN. The study met its primary endpoint, which was an improvement from baseline in the modified Neuropathy Impairment Score +7 (mNIS+7) in the treatment group compared to the placebo group, and met multiple secondary endpoints including an improvement among treated patients compared to placebo in the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QOL-DN) score and gait speed [23]. There was also a significant benefit among patisiran-treated patients compared to placebo in all major components of the COMPASS-31 scale, which assesses autonomic neuropathy [33].
2.1.2 Cardiac Outcomes
A sub-study of 126 patients from the APOLLO trial deemed to have CA based on prespecified criteria of a left ventricular (LV) wall thickness ≥ 13mm in the absence of aortic valve disease or hypertension, were analysed to assess the impact of patisiran treatment on ATTR-CM. Patisiran-treated patients had reduced LV wall thickening, increased LV end-diastolic volume, and more favourable change in global longitudinal strain (GLS) across follow-up compared to those on placebo. The treated group also had a significant reduction in N-terminal pro B-type natriuretic peptide (NT-proBNP) and increase in 10-m walk test gait speed compared to the placebo group [34].
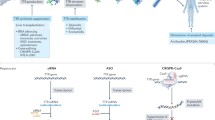
These findings were supported by a small study of 16 patients treated with patisiran and diflunisal, which demonstrated a reduction in cardiac magnetic resonance (CMR) derived extracellular volume (ECV) (Fig. 1), serum TTR and NT-proBNP, and increase in 6-min walk (6MWT) test distance after 12 months of treatment. Serial bone scintigraphy demonstrated a reduction in cardiac uptake following treatment (Fig. 2). Although the reduced cardiac uptake unequivocally indicated a favourable biological effect of patisiran, the dynamics and kinetics of radiotracer binding to bones, soft tissues and the myocardium differ, and a change in any of these compartments will affect the appearance and calculation of proportionate cardiac uptake. Reduced cardiac uptake on bone scintigraphy should be supported by improvements in other cardiac imaging modalities, cardiac biomarkers and/or functional measures before they are ascribed exclusively to a decrease in the CA burden [35].
Cardiac magnetic resonance imaging demonstrating cardiac regression following 3 years of treatment with patisiran. Following 3 years of treatment there has been a reduction in the amount of subendocardial late gadolinium enhancement and a reduction in the extracellular volume. Cine imaging sequence acquired to capture motion, ECV extracellular volume, LGE late gadolinium enhancement
The APOLLO-B trial is a phase 3 randomised controlled trial designed to assess patisiran efficacy in the treatment of ATTR-CM. This trial enrolled patients with ATTRwt-CM or ATTRv-CM, evidence of heart failure, and a serum NT-proBNP between 300 and 8500 ng/L followed for 12 months [36]. APOLLO-B met its primary endpoint with a reduction in the decline in 6MWT distance among treated patients compared to placebo across 12 months. However, the study results are yet to be published, and therefore further detail is required before formal conclusions can be drawn [37].
2.1.3 Safety Profile
Patisiran is administered alongside premedication with dexamethasone, paracetamol, ranitidine and an antihistamine to reduce the risk of infusion-related reactions. In the phase 1 trial with patisiran, there were mild/moderate infusion-related reactions in 21% of subjects, with mild reactions resolving spontaneously and moderate reactions resolving by temporarily stopping administration and then continuing the infusion at a slower rate [31]. Patisiran effectively induced TTR knockdown, and continued use for > 18 months was associated with vitamin A reduction of 62%, reflecting the function of TTR as a carrier of retinol-binding protein that transports vitamin A. Hence, all patients receiving patisiran are routinely prescribed vitamin A supplementation (2500 IU per day) [29]. In the APOLLO phase 3 trial there were no significant differences in adverse event rates between patients on patisiran and placebo, and no eye complications were observed. Common adverse events that occurred more frequently in the patisiran group were peripheral oedema and infusion-related reactions, the latter manifesting as back pain, flushing, abdominal pain and/or nausea. The incidence of serious adverse events was low. There were seven deaths in the patisiran group, all deemed to be consistent with the natural course of ATTRv amyloidosis and not related to patisiran (Table 1) [23].
2.2 Revusiran
Revusiran is another siRNA that targets mRNA coding for TTR production. Revusiran is conjugated to N-acetylgalactosamine (GalNAc), which promotes the interaction with the asialoglycoprotein receptor, widely expressed on hepatocytes. A phase 1 trial in healthy volunteers demonstrated that revusiran was well tolerated, and treatment resulted in TTR knockdown from baseline of 50% following a single dose, and 90% following multiple doses [38]. The ENDEAVOUR trial was a phase 3, randomised, placebo-controlled, double-blind trial of 206 ATTRv-CM patients, designed to assess revusiran efficacy. The study was terminated prematurely due to safety concerns after 13% of patients receiving revusiran died, compared with 3% receiving placebo, during a median follow-up period of 7 months. Post hoc analysis found that those who died were older (aged ≥ 75 years), and had more advanced heart failure at baseline compared to those who were alive throughout the study period. Although a clear causative mechanism for the mortality imbalance could not be identified, it remains possible that revusiran may have contributed such that further development of this agent was discontinued (Table 1) [39].
2.3 Vutrisiran
Vutrisiran is another siRNA that utilises a GalNAc conjugate delivery platform. The utilization of enhanced stabilisation chemistry allows subcutaneous administration of smaller doses with longer dosing intervals compared to revusiran. In a phase 1 study of healthy volunteers, vutrisiran was rapidly absorbed with peak plasma concentrations reached at 3–5 h. Vutrisiran achieved a dose-dependent TTR knockdown; a single 25 mg subcutaneous dose resulted in a maximum TTR reduction of 80%, which was sustained for 90 days [40].
2.3.1 Neurological Outcomes
The HELIOS-A trial was an open-label, multicentre study that randomised 164 ATTRv-PN patients to vutrisiran or patisiran, and compared outcomes to an external placebo group from the APOLLO trial. At 18 months of follow-up, vutrisiran treatment resulted in an improvement in the mNIS+7 and Norfolk QOL-DN scores when compared to placebo. TTR knockdown with vutrisiran occurred within 3 weeks and was sustained throughout the 18 month study period. Vutrisiran produced a mean peak TTR knockdown of 88%, mean trough TTR knockdown of 81%, and was non-inferior to patisiran [41]. The findings from HELIOS-A resulted in FDA and European Medicines Agency (EMA) approval of vutrisiran for the treatment of ATTRv-PN in 2022 [42].
2.3.2 Cardiac Outcomes
The HELIOS-B trial is an ongoing phase 3, randomised, placebo-controlled, double-blind trial designed to evaluate the efficacy and safety of vutrisiran in 665 ATTR-CM patients (variant and wild-type). The primary end point is a composite of all-cause mortality, cardiovascular hospitalisations, and urgent heart failure hospital visits, while secondary end-points include change from baseline in 6MWT, NT-proBNP, mean LV wall thickness, GLS and Kansas City Cardiomyopathy Questionnaire Overall Summary score (KCCQ-OS) [43].
2.3.3 Safety Profile
Vutrisiran is well tolerated and has an acceptable safety profile. In contrast to patisiran, patients do not require premedication, although all patients require vitamin A supplementation. The majority of adverse events in the phase 1 trial of vutrisiran were mild, and included nasopharyngitis, headache and injection site reactions. A transient asymptomatic subclinical elevation of alanine aminotransferase (ALT) (ALT < 3 times upper limit of normal) was observed in some patients prescribed ≥ 100 mg, and one patient prescribed 200 mg developed a clinically significant asymptomatic transaminitis (ALT > 3 times upper limit of normal), associated with a temporary rise in creatine kinase thought to be secondary to excessive physical activity [41]. The HELIOS-A trial also reported encouraging safety and tolerability, with the majority of adverse events being mild or moderate. There were two deaths in the vutrisiran arm and three in the patisiran arm. An additional patient discontinued vutrisiran treatment due to a serious adverse event. Serious adverse events that resulted in death/discontinuation were COVID-19 pneumonia, acute heart failure, and iliac artery occlusion, none of which were considered related to treatment. Only two serious adverse events were attributed to vutrisiran—dyslipidaemia and urinary tract infection (Table1) [41].
3 Anti-sense Oligonucleotides
Anti-sense oligonucleotides (ASOs) are composed of a single strand, typically comprising 16–20 synthetic nucleotides, which binds to complementary mRNA to induce degradation of the mRNA through a variety of mechanisms, such as a sterically blocking ribosome attachment or promoting endogenous ribonuclease H1-mediated degradation [44,45,46]. Unlike siRNAs, ASOs are able to enter cells without the use of a transfection reagent [44, 47].
3.1 Inotersen
Inotersen is a 2´-O-methoxyethyl-modified ASO that binds to the 3´ untranslated portion of the complementary mRNA, promoting ribonuclease H1-mediated mRNA degradation. Inotersen was the first ASO developed for ATTR amyloidosis, and approved by the FDA and EMA in 2018 for the treatment of ATTRv-PN [48,49,50]. In a randomised, placebo-controlled phase 1 study in 65 healthy volunteers, inotersen was administered at varying doses over a 4-week period. Inotersen lowered TTR levels in a dose-dependent manner, with 300 mg achieving a maximum TTR knockdown of 96%. Inotersen has a long-lasting effect, with trial participants showing a mean TTR knockdown of 30% 10 weeks after receiving their last dose [51].
3.1.1 Neurological Outcomes
The NEURO-TTR trial was a phase 3, randomised, placebo-controlled, double-blind study of 225 patients that assessed the efficacy and safety of inotersen in ATTRv-PN. The study met its primary endpoints with treated patients achieving a significant reduction in the decline from baseline in mNIS+7 and Norfolk QOL-DN scores compared to placebo patients. A steady state of circulating TTR concentration was reached at 13 weeks, at which point the mean TTR knockdown from baseline was 84% [24].
A total of 109 patients who completed the NEURO-TTR trial participated in an open-label extension study, intended to assess the long-term efficacy and safety of inotersen over 3 years. The group who continued on inotersen (n = 70) demonstrated sustained benefit, as measured by mNIS+7, Norfolk QOL-DN and 36-Item Short-Form Health Survey (quality-of-life questionnaire), when compared to the placebo-inotersen (n = 39) group, while the placebo-inotersen group demonstrated better neurological outcomes than those predicted by natural history data [52].
3.1.2 Cardiac Outcomes
In the whole study population and 108 patients with CA enrolled into the NEURO-TTR trial, inotersen therapy did not result in any significant change in echocardiographic parameters [24]. A small study of ATTR-CM patients (n = 30) demonstrated that 12 months of inotersen therapy stabilised cardiac disease as measured by 6MWT, GLS and CMR-derived wall thickness and LV mass [53]. Another small study of ATTR-CM patients (n = 33) demonstrated that inotersen resulted in a decreased LV mass and increased 6MWT at both 2 and 3 years [54]. The treatment of ATTR-CM with inotersen is currently being evaluated in an open-label phase 2 trial of 50 patients. The study will assess changes in echocardiographic GLS, and CMR-derived LV mass and ECV at baseline, 6, 12 and 24 months [55].
3.1.3 Safety Profile
In the NEURO-TTR trial the majority of adverse events were mild/moderate, and included nausea, fever, headache, injection site reactions and thrombocytopenia, which occurred in at least 20% of patients receiving inotersen. Notably adverse events were the most common reason for discontinuation and resulted in withdrawal of 14% patients in the inotersen group. There were five deaths in the study (all in the inotersen group), four of which were attributable to disease progression. One patient in the trial had a fatal intracranial haemorrhage secondary to inotersen-induced thrombocytopenia. Indeed, 54% of patients receiving inotersen developed platelet counts of < 140,000/mm3, three of whom had platelet counts < 25,000/mm3. Inotersen-induced immune-mediated thrombocytopenia is thought to be the most likely mechanism for this phenomenon. Thrombocytopenia was associated with the presence of anti-platelet antibodies and recovered with glucocorticoid therapy [24]. However, regular platelet monitoring during treatment with inotersen is essential [48, 50]. Glomerulonephritis occurred in three patients receiving inotersen, all of whom carried the p.(Val50Met) TTR variant. A crescentic glomerulonephritis was identified on renal biopsy in each case, and two of three were successfully treated with immunosuppression, whilst the remaining patient did not receive immunosuppressive therapy due to a late presentation, and required dialysis [24]. All patients receiving inotersen must therefore undergo frequent monitoring of renal function [48, 50]. All patients in the NEURO-TTR trial received vitamin A supplementation, and although no clinical manifestations of vitamin A deficiency were reported, vitamin A supplementation remains a requirement [24, 50].
Similar results were observed in the open-label extension, with 22% discontinuing treatment following an adverse event, although only 4% were considered related to inotersen. Adverse events included thrombocytopenia, chills, hypertension and a transaminitis that resolved on discontinuing therapy. No cases of glomerulonephritis were reported in the open-label extension study. There were 16 deaths, none of which were attributed to inotersen (Table 1) [52].
3.2 Eplontersen
Eplontersen is an ASO with a similar design and an identical sequence to inotersen, and is conjugated to a triantennary GalNAc moiety. This moiety acts as a ligand to facilitate receptor-mediated hepatocyte uptake via the high-capacity asialoglycoprotein receptors [56, 57]. Once internalised, the triantennary GalNAc is metabolised via an endocytic pathway to release the ASO, allowing it to bind the target mRNA [58]. The superior ligand-conjugated delivery system resulted in eplontersen being 50-fold more potent than inotersen at reducing TTR expression in human hepatocytes. These results were replicated in transgenic mice expressing the p.(Ile104Ser) variant and demonstrated that eplontersen induced a 28-fold more potent TTR knockdown than inotersen. In a randomised, placebo-controlled, phase 1 study of 45 healthy volunteers who received a single dose of eplontersen (120 mg) or multiple doses of 45 mg, 60 mg or 90 mg of eplontersen or placebo, eplontersen was rapidly absorbed into the systemic circulation. A single dose of eplontersen (120 mg) resulted in maximum TTR knockdown of 86%, while multiple doses resulted in maximum TTR knockdown of 86%, 91% and 94%, respectively [59].
3.2.1 Neurological Outcomes
The NEURO-TTRansform trial is a phase 3, multicentre, randomised trial designed to assess the efficacy and safety of eplontersen in the treatment of ATTRv-PN. The trial is currently ongoing and aims to recruit 140 patients who will be randomised to receive eplontersen once every 4 weeks or inotersen once weekly, while the NEURO-TTR trial placebo group will serve as an external control group. The co-primary endpoints are the change from baseline in mNIS+7 and percentage change in serum TTR, and the secondary efficacy endpoint is the change from baseline in the Norfolk QOL-DN score. The interim 35-week analysis demonstrated a significant reduction in both the co-primary endpoint and the secondary endpoint compared to the external placebo group, and the complete trial results are expected in 2024 [60,61,62].
3.2.2 Cardiac Outcomes
The CARDIO-TTRansform trial is a phase 3, multicentre, double-blind, randomised, placebo-controlled trial designed to assess the efficacy and safety of eplontersen in ATTR-CM. The trial is ongoing and aims to recruit 1500 patients who will be randomised to receive eplontersen or placebo. The primary end-point is a composite of cardiovascular mortality and recurrent cardiovascular events occurring within 140 weeks of follow-up. Secondary endpoints include change in 6MWT and KCCQ-OS at 120 weeks. The trial organisers will run a CMR sub-study to assess the effect of eplontersen on amyloid burden via CMR measurement of ECV. One aspect of the CARDIO-TTRansform trial is that patients treated with tafamidis are also eligible for inclusion, and this may enable post hoc subgroup analysis to assess the efficacy of combination therapy [63].
3.2.3 Safety Profile
There were no safety concerns during the phase 1 study. Eplontersen was well tolerated with no drug discontinuations. Commonly reported adverse events were headache (n = 2), transient increased ALT (n = 4) and creatinine kinase (n = 4). Two patients had an ALT rise > 3 times the upper limit of normal, but the transaminitis reversed after patients received their last dose without any sequelae. No injection-site reactions were reported. All patients are prescribed vitamin A due to the theoretical risk of vitamin A deficiency, but thus far no clinical manifestations of such have been reported (Table 1) [59].
4 CRISPR/Cas9-Based in vivo Genome Editing
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) were discovered as part of the archaea and bacterial immune systems [64, 65]. The CRISPR sequences are transcribed into small RNAs capable of guiding the system to complementary DNA sequences. The Cas9 endonuclease subsequently binds the DNA, and cleaves it, knocking out the target gene. Harnessing this naturally occurring biological mechanism as a potential ‘cut and paste’ tool for gene sequences has established CRISPR/Cas9-based in vivo gene editing as an exciting novel therapeutic strategy. Utilisation of modified Cas9 also allows the upregulation of target gene expression. CRISPR-Cas9 technology is being investigated as a potential treatment for multiple gene-mediated disease processes [66].
ATTR amyloidosis is the prototypic model disease for targeted in vivo genome-editing therapy. The TTR protein is coded for by a single gene, and circulating TTR is exclusively synthesised by the liver. Targeted hepatocyte delivery of a gene-editing therapy has the potential to completely ‘switch off’ TTR production, and prevent subsequent formation of pathogenic ATTR amyloid fibrils [67]. siRNA- and ASO-based therapies have demonstrated that TTR knockdown through gene silencing is clinically beneficial in ATTR amyloidosis. Furthermore, there is considerable overlap in the physiological role of TTR as a carrier protein for vitamin A and thyroxine. TTR knockdown does not appear to effect thyroid function and vitamin A supplementation, has thus far prevented any potential clinical sequelae from TTR deficiency [23, 24].
NTLA-2001 is a CRISPR-Cas9-based genome-editing therapy, transported by a lipid nanoparticle mediated delivery system. Following a single administration, NTLA-2001 resulted in significant editing of the mouse TTR-gene and 96% reduction in serum TTR levels that lasted at least 12 months. These results were replicated in various animal models including cynomolgus monkeys and transgenic mice carrying the human p.(Val50Met) variant with no adverse events [66].
A two-part open-label, single-dose, phase 1 multicentre study in ATTRv-PN and ATTR-CM patients is ongoing to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of NTLA-2001, the latter by measurement of serum TTR concentration. Interim results from the dose-ascending phase of the study showed that NTLA-2001 produced a dose-dependent TTR knockdown. Patients were pre-medicated with corticosteroids and antihistamines. The infusion was completed in all patients, without interruption, and few serious adverse events were reported. The most commonly reported treatment-related adverse events were minor infusion-related reactions manifesting as nausea, headache, chills and rhinorrhea. Patients who received therapeutic doses of NTLA-2001 had mean TTR knockdown from baseline of at least 90%, which was sustained through to last follow-up. TTR knockdown with NTLA-2001 is expected to be permanent [25]. The dose expansion phase in both the polyneuropathy and cardiomyopathy arms of the trial is ongoing (Table 1) [68].
5 Future Perspectives
5.1 Long-Term Safety of TTR Depletion
Although in the short to medium term TTR knockdown through gene silencing and editing approaches appears safe, further research is needed to establish the long-term effects of TTR deficiency [67]. Traditionally it was thought the physiological role of TTR was confined to transport of vitamin A and thyroxine. However, there is some evidence that suggests TTR may have other functions in the central nervous system. Complete TTR knockdown in 5-month-old mice resulted in memory impairment compared with age-matched mice [69], and the absence of TTR in rats accelerated the decline in cognitive performance associated with aging [70]. Such findings led to the suggestion that TTR may protect against the neurodegeneration that occurs in Alzheimer’s disease, a condition characterised by brain accumulation of amyloid-β plaques. In vitro studies demonstrated that when added to cerebrospinal fluid of patients and controls, amyloid-β was sequestered by TTR [71]. Mouse models have demonstrated the presence of amyloid-β plaques results in hippocampal upregulation of TTR, suggesting this may represent a neuroprotective response [72]. Anti-TTR antibody infusion resulted in loss of the neuroprotective effects of TTR, and subsequent increased amyloid-β plaque formation, neuronal loss and apoptosis [73].
Additional animal studies have suggested a possible neuroprotective effect of TTR in ischaemia. In mouse models with surgically induced brain ischaemia following middle cerebral artery occlusion those with complete TTR knockdown had a significantly larger infarct and greater degree of cerebral oedema [74], while the subsequent incubation of hippocampal neurones with recombinant TTR demonstrated that TTR significantly increased neurite growth [75]. The neuroprotective role of TTR is not limited to animal studies. A large clinical study of 585 patients with a cerebral infarction demonstrated that serum TTR was an independent predictor of good clinical outcomes [76].
TTR may also have a role in metabolism. Animal models demonstrated that central TTR plays a role in modulating food intake and body weight. Intracerebroventricular TTR administration in normal growing rats resulted in decreased food intake and reduced body weight. There were also decreased levels of neuropeptide Y (a hormone well known to stimulate appetite) in the dorsomedial hypothalamus and paraventricular nucleus. These findings suggest TTR may also have anorectic properties [77]. Circulating TTR plays a role in maintaining serum levels of retinol-binding protein 4 (RBP4), a circulating adipokine that has been linked to insulin resistance, type II diabetes and metabolic syndrome [78]. TTR binds RBP4 and prevents renal clearance. Not only are serum TTR levels elevated in insulin-resistant mice [79] and some insulin-resistant humans [80], but treatment of obese mice with a TTR ASO reduces circulating RBP4, and in turn reduces insulin levels and increases insulin sensitivity [81]. Notably, however, there has been no evidence thus far of cognitive decline or abnormality, or indeed metabolic abnormality in patients receiving gene silencers for more than 5 years and, importantly, TTR in the cerebrospinal fluid, which is synthesised in the choroid plexus and retinal pigment epithelium, is unaffected by NTLA-2001 therapy, which specifically targets liver production of TTR.
Specific concerns regarding off-target gene editing and unintended gene sequence deletions or insertions with CRISPR/Cas9 therapy have been raised [82, 83]. However, complex computational modelling and biochemical assays, including in vitro with human cells and in vivo in animal models have shown no evidence of off-target editing with NTLA-2001 and DNA structural variants induced by NTLA-2001 were all related to end-joining at the TTR target site. The risk of off-target editing with NTLA-2001 is therefore deemed to be low; however, patients who receive this gene-editing therapy will require long-term follow-up [25].
ATTR amyloidosis remains a progressive, debilitating and ultimately fatal disease, for which gene silencers and gene-editing therapies show great promise. Whilst the prognosis of patients with ATTR amyloidosis in the short to medium term has already been improved by these novel therapies, we must remain vigilant since long-term safety remains unknown. Careful and thorough monitoring of patients receiving these therapies, alongside clinical trials with prolonged follow-up periods, are ongoing.
5.2 Monitoring the Cardiac Response to Treatment
The majority of large clinical trials have assessed the efficacy of gene silencers in ATTRv-PN, and primary end-points have been changes in standardised measures of neuropathic disease severity such as mNIS+7 and Norfolk QOL-DN scores [23, 24]. There is growing interest in use of gene silencers to treat ATTR-CM, and hence an increasing need to develop standardised measures of cardiac response. The ATTR-ACT trial of tafamidis used a composite hierarchical primary endpoint of all-cause mortality and cardiovascular hospitalisations and secondary endpoints of change in 6MWT and KCC-QS score [21]. Although this trial did meet its primary endpoint, none of the changes assessed were a direct measure of change in CA burden and all were subject to other confounders.
NT-proBNP has long been used in heart failure to monitor treatment response, and been extensively utilised in monitoring chemotherapy response in cardiac AL amyloidosis [84]. However, changes in NT-proBNP represent the final common pathway of several mechanisms such as renal impairment, fluid status, neurohormonal activation and changes in cardiac function [85], and therefore are not necessarily an accurate reflection of change in CA burden [86]. High-sensitivity troponins measure ongoing myocyte damage and have demonstrated utility in various acute cardiac conditions, such as myocardial infarction and myocarditis [87], as well as contributing to the stratification of cardiac AL amyloidosis patients [88]. High-sensitivity means even small changes in circulating levels reflect differences in disease activity [87]. However, the use of high-sensitivity troponins in monitoring cardiac treatment response requires further investigation and validation in large-scale studies.
This unmet clinical need of measuring cardiac treatment response according to myocardial amyloid burden resulted in a growing interest in cardiac imaging. Echocardiography remains the most widely available and inexpensive imaging modality, and has demonstrated utility in cardiac AL amyloidosis patients, whereby GLS has been associated with the CA burden [89], and improvements in GLS, in response to chemotherapy, independently predicts survival [90]. GLS has been utilised in the ATTR-CM population, and stabilisation following treatment with diflunisal [91] and improvement following treatment with patisiran have been reported [34]. However, echocardiographic measures are subject to significant intra- and inter-observer variability, which is an important limitation when only small measurement variations are expected [92]. Improvements in precision could play a central role in monitoring treatment response, and be assisted by the emergence of data science [11]. The utility of artificial intelligence and more specifically convolutional neural networks has shown great promise in cardiovascular imaging [93, 94]. Elimination of manual operator contouring results in robust measures of structural and functional parameters, with a significant improvement in precision [93, 95]. These advances in automation may enable more accurate measurement of cardiac response to treatment by echocardiography.
CMR with the utilisation of multi-parametric mapping techniques is rapidly emerging as an important tool in the assessment ATTR-CM. Late gadolinium enhancement (LGE) demonstrates the continuum of CA, from subendocardial LGE to increasing transmurality as the disease progresses [96]. ECV measurement enable myocyte and extracellular compartment to be measured separately. With amyloidosis being the exemplar interstitial disease, and amyloid fibril deposition resulting in extracellular expansion, myocardial ECV quantification is an accurate surrogate measure of CA burden [4, 97, 98]. In cardiac AL amyloidosis, ECV has been able to accurately measure disease severity, and improve patient stratification [86, 99], while changes in ECV in response to treatment accurately predict outcome even after adjusting for known predictors such as changes in NT-proBNP and GLS [100]. ECV mapping allows detection of changes in tissue characterisation that are likely to occur, ahead of changes in conventional structural and functional echocardiographic parameters. To date, only one study of ATTR-CM patients has demonstrated ECV regression [35], but if these findings are replicated on a larger scale, ECV mapping may become the gold standard for monitoring the effects of treatment on amyloid burden (Fig. 1).
It is noteworthy that reduced cardiac uptake has been reported on bone scintigraphy following combination treatment with patisiran and diflunisal. These patients had evidence of a qualitative reduction in cardiac uptake on planar imaging, and quantitative reduction in the percentage of myocardial radiotracer uptake on single-photon emission computed tomography (SPECT). However, changes in cardiac uptake can be influenced by changes in the dynamics and kinetics of bone tracers that bind to several ‘compartments’ including bones, soft tissues and the myocardium. Therefore, bone scintigraphy alone cannot be used as a reliable measure of the cardiac response to treatment (Fig. 2) [35].
Positron emission tomography (PET) is another form of cardiac imaging with diagnostic potential in ATTR-CM. Several PET tracers, such as 18F-florbetapir, 18F-florbetaben, 18F-flutemetamol and 11C-Pittsburgh B, have been successfully used to diagnose CA, with tracers binding to amyloid fibrils with high affinity and potentially allowing quantification of amyloid burden. There is a lack of robust data to support its use in tracking treatment response, but future PET developments may lead to novel exciting measures of the cardiac response (Table 2) [101,102,103,104,105,106,107].
5.3 Combination Therapy
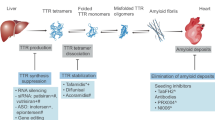
Gene silencers and editing therapies effectively induce an 80–95% knockdown of circulating plasma TTR. Following treatment with these agents, some TTR remains in the circulation that has the potential to misfold into pathogenic ATTR amyloid fibrils. More data are required to establish whether combining these agents with a TTR stabiliser such as diflunisal, tafamids or acoramidis could augment their therapeutic efficacy [21, 22, 108]. It is conceivable that simultaneously targeting both TTR production and TTR stabilisation could have a synergistic effect and result in improved patient outcomes. It is noteworthy that patisiran was used in combination with diflunisal in the only study to demonstrate cardiac ATTR amyloid regression [35]. Further large-scale studies are required to determine whether combination therapy provides additional clinical benefit in ATTR amyloidosis.
5.4 Impact of Earlier Diagnosis
Advances in cardiac imaging, alongside the increased awareness amongst clinicians has contributed to ATTR-CM patients being diagnosed earlier in the disease course. Patients now experience a shorter duration of symptoms prior to diagnosis, and have a better functional capacity and milder disease stage at the time of diagnosis [109]. The milder cardiac phenotype has translated into improved survival from the time of diagnosis, even when accounting for the impact of disease-modifying therapy. Utilisation of gene-silencing and gene-editing therapies early in the natural history of ATTR-CM may prevent the development of overt heart failure and translate into markedly better clinical outcomes, but more data are needed to guide decisions on when and in whom to initiate treatment. Importantly, changes in the clinical phenotype of patients diagnosed with ATTR-CM will influence the design of future clinical trials to evaluate the efficacy of novel agents and will likely result in a requirement for greater patient numbers and longer follow-up to ensure adequate power [109].
5.5 Comparisons Between Different Gene Silencers and Gene-Editing Therapies
The last decade has seen incredible advances in the treatment for ATTR amyloidosis. Initial treatment strategies sought to achieve TTR stabilisation, and resulted in licensing of tafamidis for ATTR-CM [21]. Although clinical trials demonstrated efficacy in slowing disease progression, tafamidis does not achieve complete TTR stabilisation in vivo and patients continue to progress while on treatment, albeit at a slower rate. The advent of gene silencers has transformed the treatment landscape. These novel therapeutic agents target TTR production and reduce the amount of circulating TTR protein. Patisiran was the first siRNA to be approved for the treatment of ATTR-PN, and can actually improve neurological outcomes [23], while early data suggest it may not only be able to stabilise cardiac disease but also induce disease regression [35]. Further advances in conjugate delivery platforms led to the development of vutrisiran, an siRNA that can be administered subcutaneously every 3 months, without the need for premedication (as opposed to the 3-weekly intravenous infusions of patisiran). Following publication of the HELLIOS-A trial results, vutrisiran was licensed for treatment of ATTR-PN and offers a convenient but equally effective alternative to patisiran [41, 42]. The HELIOS-B trial results will shed further light on the efficacy of vutrisiran for treatment of ATTR-CM [43].
The evidence for ASO-based treatments is limited to the NEURO-TTR trial, which assessed the use of once-weekly inotersen for treatment of ATTR-PN. Despite demonstrating efficacy, inotersen did not lower circulating TTR levels any more than patisiran, and in comparison, was poorly tolerated with a significant proportion of patients discontinuing therapy following adverse events. Severe adverse events included thrombocytopenia and glomerulonephritis, which, although uncommon, carried a significant risk of morbidity and mortality [24]. It is also noteworthy that mNIS+7 composite scores differ between siRNA and ASO trials, which should be considered in any attempt at comparison between studies [23, 24]. Whilst siRNAs initially seemed more promising than ASO therapy due to a better safety profile, the emergence of eplontersen may change this premise. Eplontersen is a once-monthly subcutaneous injection, administrated without the need for premedication. Initial phase 1 results have confirmed a favourable safety profile, with no reports of serious adverse events, and demonstrated an amplified potency when compared to inotersen [59]. The large-scale phase 3 NEURO-TTRansform [61] and CARDIO-TTRansform [63] trials are ongoing and are designed to assess efficacy of eplontersen in ATTR-PN and ATTR-CM respectively.
Perhaps the most exciting treatment development is the breakthrough of CRISPR/Cas9 in vivo gene editing and its ability to specifically and effectively target the TTR gene. The prospect of a single-dose treatment for ATTR amyloidosis may be the first step towards a ‘one and done’ cure for this debilitating and fatal disease [25].
6 Conclusions
In summary, the emergence of novel gene-silencing and gene-editing therapies have rapidly expanded the armamentarium of treatments available for ATTR amyloidosis. Targeting TTR production has resulted in tremendous improvements in patient outcomes. With the results of large-scale clinical trials expected over the next few years, a further expansion of medicines licensed for treatment of ATTR amyloidosis is very likely. Whilst there do not appear to be any clinical consequences in the short to medium term of depletion of circulating TTR, the long-term effects of these drugs both in terms of efficacy and safety remain to be determined.
References
Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–54.
Lachmann HJ, Hawkins PN. Systemic amyloidosis. Curr Opin Pharmacol. 2006;6:214–20.
Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med (Lond). 2018;18:s30–5.
Fontana M, Ćorović A, Scully P, Moon JC. Myocardial amyloidosis: the exemplar interstitial disease. JACC Cardiovasc Imaging. 2019;12:2345–56.
Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res Cardiovasc Res. 2022. https://doi.org/10.1093/cvr/cvac119.
Ravichandran S, Lachmann HJ, Wechalekar AD. Epidemiologic and Survival Trends in Amyloidosis, 1987–2019. N Eng J Med. 2020;382:1567–8.
Lauppe RE, Liseth Hansen J, Gerdesköld C, Rozenbaum MH, Strand AM, Vakevainen M, et al. Original research: nationwide prevalence and characteristics of transthyretin amyloid cardiomyopathy in Sweden. Open Heart. 2021;8:1755.
Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–9.
Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, Saraiva MJM, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23:209–13.
Patel RK, Ioannou A, Razvi Y, Chacko L, Venneri L, Bandera F, et al. Sex differences among patients with transthyretin amyloid cardiomyopathy—from diagnosis to prognosis. Eur J Heart Fail. 2022. https://doi.org/10.1002/ejhf.2646.
Ioannou A, Patel R, Gillmore JD, Fontana M. Imaging-guided treatment for cardiac amyloidosis. Curr Cardiol Rep. 2022;1:1–12.
Ioannou A, Patel RK, Razvi Y, Porcari A, Knight D, Martinez-Naharro A, et al. Multi-imaging characterization of cardiac phenotype in different types of amyloidosis. JACC Cardiovasc Imaging. 2022. https://doi.org/10.1016/j.jcmg.2022.07.008.
Carr AS, Pelayo-Negro AL, Evans MRB, Laurà M, Blake J, Stancanelli C, et al. A study of the neuropathy associated with transthyretin amyloidosis (ATTR) in the UK. J Neurol Neurosurg Psychiatry. 2016;87:620–7.
Yanagisawa A, Ueda M, Sueyoshi T, Okada T, Fujimoto T, Ogi Y, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28:201–7.
Geller HI, Singh A, Alexander KM, Mirto TM, Falk RH. Association between ruptured distal biceps tendon and wild-type transthyretin cardiac amyloidosis. JAMA. 2017;318:962–3.
Coelho T, Maurer MS, Suhr OB. THAOS—The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76.
Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014;35:E2403–12.
Waddington-Cruz M, Wixner J, Amass L, Kiszko J, Chapman D, Ando Y, et al. Characteristics of patients with late- vs. early-onset Val30Met transthyretin amyloidosis from the transthyretin amyloidosis outcomes survey (THAOS). Neurol Ther. 2021;10:753–66.
Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22:171–4.
Arruda-Olson AM, Zeldenrust SR, Dispenzieri A, Gertz MA, Miller FA, Bielinski SJ, et al. Genotype, echocardiography, and survival in familial transthyretin amyloidosis. Amyloid. 2013;20:263–8.
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–16.
Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67.
Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Eng J Med. 2018;379:11–21.
Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31.
Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385:493–502.
Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:101.
Caplen NJ, Mousses S. Short interfering RNA (siRNA)-mediated RNA interference (RNAi) in human cells. Ann N Y Acad Sci. 2003;1002:56–62.
Friedrich M, Aigner A. Therapeutic siRNA: state-of-the-art and future perspectives. BioDrus. 2022;36:549–71.
Urits I, Swanson D, Swett MC, Patel A, Berardino K, Amgalan A, et al. A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther. 2020;9:301–15.
Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421–46.
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29.
Schmidt HH, Wixner J, Planté-Bordeneuve V, Muñoz-Beamud F, Lladó L, Gillmore JD, et al. Patisiran treatment in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy after liver transplantation. Am J Transplant. 2022;22:1646–57.
González-Duarte A, Berk JL, Quan D, Mauermann ML, Schmidt HH, Polydefkis M, et al. Analysis of autonomic outcomes in APOLLO, a phase III trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. J Neurol. 2020;267:703–12.
Solomon SD, Adams D, Kristen A, Grogan M, González-Duarte A, Maurer MS, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139:431–43.
Fontana M, Martinez-Naharro A, Chacko L, Rowczenio D, Gilbertson JA, Whelan CJ, et al. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging. 2021;14:189–99.
APOLLO-B: A Study to Evaluate Patisiran in Participants With Transthyretin Amyloidosis With Cardiomyopathy (ATTR Amyloidosis With Cardiomyopathy). ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03997383. Accessed 11 Sep 2022.
Topline Results from APOLLO-B Phase 3 Study of Patisiran. alnylampharmaceuticalsinc.gcs-web.com. https://alnylampharmaceuticalsinc.gcs-web.com/static-files/dc61b882-9346-4394-9f5c-dd322727742b. Accessed 11 Sep 2022.
Zimmermann TS, Karsten V, Chan A, Chiesa J, Boyce M, Bettencourt BR, et al. Clinical proof of concept for a novel hepatocyte-targeting GalNAc-siRNA conjugate. Mol Ther. 2017;25:71–8.
Judge DP, Kristen AV, Grogan M, Maurer MS, Falk RH, Hanna M, et al. Phase 3 multicenter study of revusiran in patients with hereditary transthyretin-mediated (hATTR) amyloidosis with cardiomyopathy (ENDEAVOUR). Cardiovasc Drugs Ther. 2022;34:357–70.
Habtemariam BA, Karsten V, Attarwala H, Goel V, Melch M, Clausen VA, et al. Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting n-acetylgalactosamine–small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther. 2021;109:372–82.
Adams D, Tournev IL, Taylor MS, Coelho T, Planté-Bordeneuve V, Berk JL, et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2022;23:1–9.
Keam SJ. Vutrisiran: first approval. Drugs. 2022;82:1419–25.
HELIOS-B: A Study to Evaluate Vutrisiran in Patients With Transthyretin Amyloidosis With Cardiomyopathy-Full Text View-ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04153149. Accessed 11 Sep 2022.
Hayashi Y, Jono H. Recent advances in oligonucleotide-based therapy for transthyretin amyloidosis: clinical impact and future prospects. Biol Pharm Bull. 2018;41:1737–44.
Wu H, Lima WF, Zhang H, Fan A, Sun H, Crooke ST. Determination of the role of the human RNase H1 in the pharmacology of DNA-like antisense drugs. J Biol Chem. 2004;2004(279):17181–9.
Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–93.
Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:1–16.
Tegsedi | Highlights of prescribing information. Dailymed.nlm.nih.gov. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8513207e-b55f-417b-9473-af785146a543. Accessed 12 Sep 2022.
Tegsedi | European Medicines Agency. Ema.europa.eu/en. https://www.ema.europa.eu/en/medicines/human/EPAR/tegsedi#authorisation-details-section. Accessed 11 Sep 2022.
Keam SJ. Inotersen: First Global Approval. Drugs. 2018;78:1371–6.
Ackermann EJ, Guo S, Benson MD, Booten S, Freier S, Hughes SG, et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides. Amyloid. 2016;23:148–57.
Brannagan TH, Coelho T, Wang AK, Polydefkis MJ, Dyck PJ, Berk JL, et al. Long-term efficacy and safety of inotersen for hereditary transthyretin amyloidosis: NEURO-TTR open-label extension 3-year update. J Neurol. 2022;269:6416–27.
Benson MD, Dasgupta NR, Rissing SM, Smith J, Feigenbaum H. Safety and efficacy of a TTR specific antisense oligonucleotide in patients with transthyretin amyloid cardiomyopathy. Amyloid. 2017;24:219–25.
Dasgupta NR, Rissing SM, Smith J, Jung J, Benson MD. Inotersen therapy of transthyretin amyloid cardiomyopathy. Amyloid. 2020;27:52–8.
24 Month Open Label Study of the Tolerability and Efficacy of Inotersen in TTR Amyloid Cardiomyopathy Patients. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03702829. Accessed 12 Sep 2022.
Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–807.
Tanowitz M, Hettrick L, Revenko A, Kinberger GA, Prakash TP, Seth PP. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 2017;45:12388–400.
Shemesh CS, Yu RZ, Gaus HJ, Greenlee S, Post N, Schmidt K, et al. Elucidation of the biotransformation pathways of a Galnac3-conjugated antisense oligonucleotide in rats and monkeys. Mol Ther Nucleic Acids. 2016;5: e319.
Viney NJ, Guo S, Tai LJ, Baker BF, Aghajan M, Jung SW, et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail. 2021;8:652–61.
Coelho T, Ando Y, Benson MD, Berk JL, Waddington-Cruz M, Dyck PJ, et al. Design and rationale of the global phase 3 NEURO-TTRansform study of antisense oligonucleotide AKCEA-TTR-LRx (ION-682884-CS3) in hereditary transthyretin-mediated amyloid polyneuropathy. Neurol Ther. 2021;10:375–89.
NEURO-TTRansform: A Study to Evaluate the Efficacy and Safety of Eplontersen (Formerly Known as ION-682884, IONIS-TTR-LRx and AKCEA-TTR-LRx) in Participants With Hereditary Transthyretin-Mediated Amyloid Polyneuropathy. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04136184. Accessed 11 Sep 2022.
Ionis presents positive results from Phase 3 NEURO-TTRansform study at International Symposium on Amyloidosis | Ionis Pharmaceuticals, Inc. https://ir.ionispharma.com/news-releases/news-release-details/ionis-presents-positive-results-phase-3-neuro-ttransform-study. Accessed 22 Dec 2022.
CARDIO-TTRansform: A Study to Evaluate the Efficacy and Safety of Eplontersen (Formerly Known as ION-682884, IONIS-TTR-LRx and AKCEA-TTR-LRx) in Participants With Transthyretin-Mediated Amyloid Cardiomyopathy (ATTR CM). ClinicalTrials.gov. [cited 2022 Sep 14]. https://www.clinicaltrials.gov/ct2/show/NCT04136171. Accessed 11 Sep 2022.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–8.
Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, et al. A Single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22:2227–35.
Maurer MS. Gene editing—a cure for transthyretin amyloidosis? N Eng J Med. 2021;385:558–9.
Study to Evaluate Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of NTLA-2001 in Patients With Hereditary Transthyretin Amyloidosis With Polyneuropathy (ATTRv-PN) and Patients With Transthyretin Amyloidosis-Related Cardiomyopathy (ATTR-CM). ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04601051. Accessed 19 Sep 2022.
Sousa JC, Marques F, Dias-Ferreira E, Cerqueira JJ, Sousa N, Palha JA. Transthyretin influences spatial reference memory. Neurobiol Learn Mem. 2007;88:381–5.
Brouillette J, Quirion R. Transthyretin: a key gene involved in the maintenance of memory capacities during aging. Neurobiol Aging. 2008;29:1721–32.
Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994;91:8368–72.
Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci. 2002;22:7380–8.
Stein TD, Anders NJ, DeCarli C, Chan SL, Mattson MP, Johnson JA. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24:7707–17.
Santos SD, Lambertsen KL, Clausen BH, Akinc A, Alvarez R, Finsen B, et al. CSF transthyretin neuroprotection in a mouse model of brain ischemia. J Neurochem. 2010;115:1434–44.
Gomes JR, Nogueira RS, Vieira M, Santos SD, Ferraz-Nogueira JP, Relvas JB, et al. Transthyretin provides trophic support via megalin by promoting neurite outgrowth and neuroprotection in cerebral ischemia. Cell Death Differ. 2016;23:1749–64.
Gao C, Zhang B, Zhang W, Pu S, Yin J, Gao Q. Serum prealbumin (transthyretin) predict good outcome in young patients with cerebral infarction. Clin Exp Med. 2011;11:49–54.
Zheng F, Kim YJ, Moran TH, Li H, Bi S. Central transthyretin acts to decrease food intake and body weight. Sci Rep. 2016;6:24238.
Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–11.
Mody N, Graham TE, Tsuji Y, Yang Q, Kahn BB. Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am J Physiol Endocrinol Metab. 2008;294:E785–93.
Klöting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87.
Zemany L, Bhanot S, Peroni OD, Murray SF, Moraes-Vieira PM, Castoldi A, et al. Transthyretin antisense oligonucleotides lower circulating RBP4 levels and improve insulin sensitivity in obese mice. Diabetes. 2015;64:1603–14.
Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24:927–30.
Enache OM, Rendo V, Abdusamad M, Lam D, Davison D, Pal S, et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat Genet. 2020;52:662–8.
Merlini G, Lousada I, Ando Y, Dispenzieri A, Gertz MA, Grogan M, et al. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia. 2016;30:1979–86.
Emdin M, Passino C, Prontera C, Iervasi A, Ripoli A, Masini S, et al. Cardiac natriuretic hormones, neuro-hormones, thyroid hormones and cytokines in normal subjects and patients with heart failure. Clin Chem Lab Med. 2004;42:627–36.
Banypersad SM, Sado DM, Flett AS, Gibbs SDJ, Pinney JH, Maestrini V, et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6:34–9.
Passino C, Aimo A, Masotti S, Musetti V, Prontera C, Emdin M, et al. Cardiac troponins as biomarkers for cardiac disease. Biomark Med. 2019;13:325–30.
Dispenzieri A, Gertz MA, Kumar SK, Lacy MQ, Kyle RA, Saenger AK, et al. High sensitivity cardiac troponin T in patients with immunoglobulin light chain amyloidosis. Heart. 2014;100:383–8.
Kim D, Choi JO, Kim K, Kim SJ, Kim JS, Jeon ES. Association of left ventricular global longitudinal strain with cardiac amyloid load in light chain amyloidosis. JACC Cardiovasc Imaging. 2021;14:1283–5.
Cohen OC, Ismael A, Pawarova B, Manwani R, Ravichandran S, Law S, et al. Longitudinal strain is an independent predictor of survival and response to therapy in patients with systemic AL amyloidosis. Eur Heart J. 2022;43:333–41.
Lohrmann G, Pipilas A, Mussinelli R, Gopal DM, Berk JL, Connors LH, et al. Stabilization of cardiac function with diflunisal in transthyretin (ATTR) cardiac amyloidosis. J Card Fail. 2020;26:753–9.
Chacko L, Karia N, Venneri L, Bandera F, Passo BD, Buonamici L, et al. Progression of echocardiographic parameters and prognosis in transthyretin cardiac amyloidosis. Eur J Heart Fail. 2022;24:1700–12.
Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1317–35.
Tromp J, Seekings PJ, Hung CL, Iversen MB, Frost MJ, Ouwerkerk W, et al. Automated interpretation of systolic and diastolic function on the echocardiogram: a multicohort study. Lancet Digit Health. 2022;4:e46-54.
Howard JP, Francis DP. Machine learning with convolutional neural networks for clinical cardiologists. Heart. 2022;108:73–81.
Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–9.
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–65.
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488–97.
Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. Editor’s choice: T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244–51.
Martinez-Naharro A, Patel R, Kotecha T, Karia N, Ioannou A, Petrie A, et al. Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac363.
Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR, di Carli MF, et al. Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:1652–62.
Dietemann S, Nkoulou R. Amyloid PET imaging in cardiac amyloidosis: a pilot study using 18 F-flutemetamol positron emission tomography. Ann Nucl Med. 2019;33:624–8.
Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjö L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med. 2013;54:213–20.
Genovesi D, Vergaro G, Giorgetti A, Marzullo P, Scipioni M, Santarelli MF, et al. [18F]-Florbetaben PET/CT for differential diagnosis among cardiac immunoglobulin light chain, transthyretin amyloidosis, and mimicking conditions. JACC Cardiovasc Imaging. 2021;14:246–55.
Kircher M, Ihne S, Brumberg J, Morbach C, Knop S, Kortüm KM, et al. Detection of cardiac amyloidosis with 18 F-Florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur J Nucl Med Mol Imaging. 2019;46:1407–16.
Lee SP, Suh HY, Park S, Oh S, Kwak SG, Kim HM, et al. Pittsburgh B compound positron emission tomography in patients with AL cardiac amyloidosis. J Am Coll Cardiol. 2020;75:380–90.
Kim YJ, Ha S, Kim YL. Cardiac amyloidosis imaging with amyloid positron emission tomography: a systematic review and meta-analysis. J Nucl Cardiol. 2020;27:123–32.
Efficacy and Safety of AG10 in Subjects With Transthyretin Amyloid Cardiomyopathy. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03860935. Accessed 20 Nov 2021.
Ioannou A, Patel RK, Razvi Y, Porcari A, Sinagra G, Venneri L, et al. Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation. 2022;146:1657–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Patient consent to participate/publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Adam Ioannou, Marianna Fontana and Julian Gillmore were all involved in writing and editing the manuscript.
Conflicts of interest
Adam Ioannou does not have any conflicts of interest. Marianna Fontana is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/21/33447) and has consulting income from Intellia, Novo-Nordisk, Pfizer, Eidos, Prothena, Alnylam, Alexion, Janssen and Ionis. Julian Gillmore has consulting income from Ionis, Alexion, Eidos, Intellia, Alnylam and Pfizer.
Funding
This work did not receive any specific funding.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ioannou, A., Fontana, M. & Gillmore, J.D. RNA Targeting and Gene Editing Strategies for Transthyretin Amyloidosis. BioDrugs 37, 127–142 (2023). https://doi.org/10.1007/s40259-023-00577-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-023-00577-7