Abstract
Background
Patients with atherosclerotic cardiovascular disease who require additional low-density lipoprotein cholesterol (LDL-C) lowering despite maximally tolerated statins have a significant unmet medical need and are at increased risk of future cardiovascular events and a reduced quality of life.
Objective
We aimed to estimate the percentage of cardiovascular events avoided following treatment with a fixed-dose combination of bempedoic acid plus ezetimibe (BA+EZE FDC) versus ezetimibe (EZE) in patients with atherosclerotic cardiovascular disease receiving maximally tolerated statins across a range of baseline LDL-C levels.
Methods
A Markov cohort simulation model estimated major adverse cardiovascular events avoided over a lifetime horizon among patients with atherosclerotic cardiovascular disease and baseline LDL-C levels from 80 to >200 mg/dL. BA+EZE FDC was compared with EZE based on mean percent LDL-C reductions versus placebo reported in a phase III trial. Health outcomes for the average patient were extrapolated to a US population of 100,000 persons using evidence on contemporary LDL-C levels from the National Health and Nutrition Examination Survey.
Results
Among patients with atherosclerotic cardiovascular disease not at the LDL-C goal with maximally tolerated statins, the addition of BA+EZE FDC compared with the addition of EZE was predicted to provide incremental absolute reductions in major adverse cardiovascular events dependent on baseline LDL-C levels at the population level. For those with baseline LDL-C of 101–110 mg/dL (n = 15,237), there were 4.9% (744) fewer events predicted, while for patients with baseline LDL-C of > 200 mg/dL (n = 1689), 10.9% (184) fewer events were predicted through the addition of BA+EZE FDC versus EZE.
Conclusions
Further LDL-C reductions through the addition of BA+EZE FDC to maximally tolerated statins are predicted to reduce major adverse cardiovascular events compared with the addition of EZE. Benefits are potentially greater among those with higher starting LDL-C.
Similar content being viewed by others
Recent treatment guidelines and consensus statements recommend that based on the level of low-density lipoprotein cholesterol lowering required to reach their goal, non-statin treatments may be added to maximally tolerated statins. |
Clinicians, policy makers, and payers should understand the benefits of non-statin oral agents such as ezetimibe (EZE) and bempedoic acid (BA) alone or in a fixed-dose combination with EZE (BA+EZE fixed-dose combination) compared with EZE alone in patients who are not able to achieve their low-density lipoprotein cholesterol goal with maximally tolerated statins alone. |
Our findings suggest the addition of BA+EZE fixed-dose combination to maximally tolerated statins in patients not at the low-density lipoprotein cholesterol goal resulted in more major adverse cardiovascular events avoided compared with EZE alone over a lifetime horizon. |
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of morbidity and the number one cause of death in the USA and globally [1, 2]. By 2035, 45.1% of the US population is projected to have some form of cardiovascular disease (CVD), with total annual costs expected to reach US$1.1 trillion [3]. Guidelines from major international professional societies and consensus statements highlight the causal role of low-density lipoprotein cholesterol (LDL-C) in atherogenesis [4, 5]. Findings from meta-analyses of over 200 prospective epidemiologic cohort studies, Mendelian randomization studies, and large randomized cardiovascular (CV) outcomes trials demonstrate that there is a log-linear association between the magnitude and duration of vascular exposure to elevated LDL-C and the risk of ASCVD [6, 7]. Further evidence indicates that the magnitude of CV benefit from LDL-C lowering is independent of the means or mechanism by which it is achieved and proportional to the absolute decrease in the LDL-C level [6,7,8]. The magnitude of absolute risk reduction depends on the baseline risk of the population. In addition, the relative risk reduction in major adverse cardiovascular events (MACE) for a given absolute reduction in LDL-C is consistent, regardless of patient demographics and medical history [6, 7, 9,10,11,12,13,14].
The Centers for Disease Control estimates that 62.6 million US adults have LDL-C levels deemed too high for their CV risk level [15]. Statins are the foundation of lipid-lowering therapy, with substantial and well-documented evidence demonstrating reductions in CV events [6]. However, not all patients are able to reach risk-based LDL-C goals on only maximally tolerated statins [47, 48], defined as the highest tolerated intensity and frequency of statins (which in some cases can mean no statin at all) [16]. Patients with ASCVD who require additional LDL-C lowering on top of maximally tolerated statins represent a patient population with a very high CV risk with a significant unmet medical need for additional lipid-lowering therapies.
Currently available non-statin treatments in the USA include oral agents (ezetimibe [EZE], bempedoic acid [BA] alone or in a fixed-dose combination with EZE [BA+EZE FDC], and bile acid sequestrants) and injectable treatments (proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors) [4]. Recent treatment guidelines and consensus statements recommend that based on the level of LDL-C lowering required to reach their goal, non-statin treatments may be added to maximally tolerated statins [5, 17, 18]. In assessing the value of different treatments, clinicians, policy makers, and payers should consider the impact of treatments on clinical outcomes. The purpose of this study was to better understand the potential benefits and place in therapy for BA+EZE FDC, a newer non-statin oral agent, compared with EZE in patients who are not able to achieve their LDL-C goal with maximally tolerated statins alone.
A long-term CV outcomes trial [45] is currently underway to assess the impact of BA on CV events in patients with elevated LDL-C levels. In the interim until those results are available, using a simulation model, we estimated the lifetime projected events (i.e., MACE) avoided among patients with ASCVD who are taking maximally tolerated statins across a range of baseline LDL-C values and are receiving BA+EZE FDC or EZE. This analysis summarizes the potential lifetime clinical benefits of adding BA+EZE FDC compared with EZE alone at various baseline LDL-C levels.
2 Methods
2.1 Model Structure
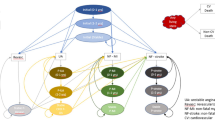
A Markov cohort simulation model with a lifetime horizon was built from a US healthcare decision-maker perspective (payer and/or clinician perspective) following best practices for decision analytic modeling in health technology evaluations [19, 20]. The structure of the model (Fig. 1) includes health states relevant for the pathway of CVD used in previous modeling efforts [21, 22]. Such health states include myocardial infarction (MI), stroke, coronary revascularization, and CV-related death (4-point MACE) [23, 24].
Modeling framework for atherosclerotic cardiovascular disease (CVD) progression. A cohort of patients with a high CVD risk (due to atherosclerotic CVD) begins treatment with ezetimibe or bempedoic acid plus ezetimibe fixed-dose combination and may stay in that state, or pass into event states such as myocardial infarction (MI), non-fatal stroke, coronary revascularization, and cardiovascular (CV) death. From there, patients may move into post-event health states where they may have a higher likelihood for additional events or death as compared with a general CVD risk. Death may occur from all-cause or event/post-event-specific mortality
Specifically, the model simulates a cohort of adults with ASCVD (who are receiving maximally tolerated statins) initiating BA+EZE FDC or EZE and either staying in the current health state or passing into the event states described above. Death could occur from non-CV reasons or from CV event/post-event-specific mortality. As patients move through the model over the course of a lifetime, they may experience CV events related to the management and treatment of high LDL-C. The cumulative sum of CV events estimate outputs such as cumulative MACE avoided (cumulative incidence).
2.2 Key Modeling Assumptions
Key modeling assumptions are related to the baseline risk of CV events, efficacy of lipid-lowering treatments on LDL-C, rate of CV events per cycle, and discontinuation/continuation of therapy. The model was calibrated using the baseline CV risk of the placebo arm (n = 13,780) of FOURIER (NCT01764633), a large multicenter, randomized, placebo-controlled trial that assessed the impact of evolocumab, a PCSK9 inhibitor, on LDL-C levels and major CV events in patients on statin therapy [25]. The FOURIER placebo arm provides contemporary (2013–16) estimates of MACE in patients for secondary prevention of ASCVD, reporting a 10-year CVD risk increase with a corresponding increase in baseline LDL-C and a 10-year CVD risk decrease as LDL-C decreases [25]. The model was calibrated to produce similar 3-year cumulative incidence estimates within 0.1%.
Efficacy parameters in the model were based on the mean percent change in LDL-C levels from baseline to 12 weeks observed in the BA+EZE FDC (vs EZE) pivotal phase III trial (Table 1) [26]. The incremental effect (difference between the two treatment scenarios) was applied to population-based LDL-C baseline distributions for persons with ASCVD in the USA, based on the National Health and Nutrition Examination Survey (NHANES) [27], to quantify effects on MI, stroke, coronary revascularization, and CV-related death using evidence from the Cholesterol Treatment Trialists’ Collaboration (CTTC) [13]. The CTTC has conducted robust patient-level meta-analyses of numerous large-scale long-term clinical trials of statin therapies to quantify the relationship between LDL-C reductions and MACE [6, 12,13,14]. As described in more detail in the Model Analyses section, 10-year CVD risk estimates were adjusted to reflect changes in LDL-C. That is, for every 10-mg/dL change in LDL-C, the CVD relative risk changed by approximately 5.6% (consistent with CTTC data) with an additional risk reduction to account for the cumulative benefit of a CV reduction over a lifetime (8.5%). Rate ratios per 1-mmol/L LDL-C reduction are consistent with CTTC evidence [13].
Outcomes were assumed to be additive for patients having more than one event in each cycle, with patients who have an event and survive continuing therapy. The same treatment effect for any given reduction in LDL-C was assumed for all subsequent CV events. Finally, discontinuation inputs were based on discontinuation rates due to adverse events in the intent-to-treat analyses of clinical trials: an 11.3% discontinuation rate was applied for the first year and a 2% discontinuation rate was assumed for each subsequent year [28].
2.3 Model Inputs
Model inputs are shown in Table 1.
2.3.1 Sources for Baseline Risk Equations for First Events, Subsequent Events, and Mortality
Model inputs for the probability of CV events varied by age and risk factors. Cardiovascular mortality was calibrated to FOURIER placebo trial evidence and subsequent excess mortality was consistently applied across treatment arms as a multiplier of 2.5 based on existing evidence of chronic CVD [25, 29, 30]. All-cause mortality was varied by age based on US life tables [31].
2.3.2 Baseline Population Characteristics, CVD Risk, and Efficacy Input Parameters
Baseline population characteristics (i.e., age, sex, baseline LDL-C) were derived from patients enrolled in the placebo arms of the BA+EZE FDC phase III trial (Table 2). To link changes in LDL-C to the risk of CV events, we used validated CV disease risk engines that vary CV risk over time [32,33,34]. Absolute reductions in LDL-C were applied to baseline CVD risk across a distribution of baseline LDL-C levels to project the reduction in CV events over time and the associated change in clinical outcomes [13]. Reductions in LDL-C levels for BA+EZE FDC and EZE were derived from the pivotal phase III trial [26] based on a placebo-adjusted reduction in LDL-C at 12 weeks (Table 2). This evidence was applied in the model as a linear effect between LDL-C lowering and the impact on CV events over time [7]. Real-world distribution of baseline LDL-C levels among persons in the USA with ASCVD was derived from NHANES [27] and data are shown in Table 3 as the percentage and number of persons per 100,000 across 10-mg/dL increments of baseline LDL-C levels.
2.3.3 Treatment Effect
Placebo-adjusted percent changes in LDL-C for BA+EZE FDC (− 38%) and EZE (− 25%) [26] were applied to NHANES-based [27] LDL-C distributions (Table 3) to estimate the impact of LDL-C lowering on MACE. Specifically, the correlation between LDL-C levels and the reduction in the rate of MACE was used to apply rate ratios to model events in each cycle. Rate ratios comparing incidence rates of events per 1-mmol/L reduction in LDL-C were derived from the CTTC meta-analysis [13]. The following rate ratios (95% confidence interval) were applied: 0.76 (0.73, 0.79) for non-fatal MI, 0.85 (0.80, 0.89) for non-fatal stroke, 0.76 (0.73, 0.79) for coronary revascularization, and 0.88 (0.84, 0.91) for any vascular death [13].
2.4 Model Analyses
The model estimated the cumulative incidence of first and subsequent CV events. Rather than presenting results with a single base-case scenario, this model analyzed findings across a range of baseline LDL-C levels commonly seen in clinical practice among patients with ASCVD.
The analysis varied LDL-C levels and the resulting 10-year baseline CVD risk associated with each starting LDL-C level displayed the impact of the starting risk on the potential benefit. The model was anchored on the base-case risk estimates from the BA+EZE FDC trial. A reduction (or increase) in the LDL-C level of 10 mg/dL was predicted to result in a reduction (or increase) in the 10-year relative risk of MACE by 5.6% to simulate overall MACE avoided based on the resulting LDL-C level and the corresponding 10-year CVD risk. Efficacy of BA+EZE FDC was assumed to be consistent across baseline levels of LDL-C, as demonstrated in the phase III trial [26]. Final results were presented as lifetime MACE avoided per 100,000 persons treated with BA+EZE FDC versus EZE, after adjusting for the population size at each LDL-C starting level.
3 Results
At the population level, Fig. 2 represents risk by the baseline LDL-C level and the distribution of persons with ASCVD within those starting levels. It shows the number of MACE avoided per 100,000 persons derived from the NHANES sample in Table 3. The largest sample includes starting levels of LDL-C of 101–110 mg/dL or approximately 15,000 people per 100,000. Incremental gains in 4-point MACE avoided in patients treated with BA+EZE FDC versus EZE alone for this sub-population include the avoidance of approximately 744 events, including 279 CV deaths, 122 MIs, 17 strokes, 139 coronary revascularizations, and 187 subsequent events. As the LDL-C at baseline increases along with the associated 10-year risk of CV events, the percentage of events avoided among all persons treated also increases. For the 15,237 patients with LDL-C baseline levels of 101–110 mg/dL, the percentage of total 4-point MACE avoided over a lifetime from treatment with BA+EZE FDC versus EZE alone is an approximately 4.9% absolute risk reduction or 744 events. As the LDL-C levels increase to more than 200 mg/dL, the percentage of total 4-point MACE avoided over a lifetime increases to an approximately 10.9% absolute risk reduction or 184 events avoided among the 1689 persons treated with BA+EZE FDC versus EZE alone.
Lifetime major adverse cardiovascular events (MACE) avoided with bempedoic acid plus ezetimibe fixed-dose combination (BA+EZE FDC) versus EZE. As the 10-year risk increases, driven largely by low-density lipoprotein cholesterol (LDL-C) at baseline, the incremental benefits in terms of 4-point MACE increase. CV cardiovascular, NHANES National Health and Nutrition Examination Survey
4 Discussion
We adapted a Markov model to predict the incremental impact of treatment with BA+EZE FDC on CV outcomes over a lifetime horizon, across 10-mg/dL increments in LDL-C levels among patients not reaching their LDL-C goal while taking maximally tolerated statins. This simulation model evaluates the impact of different non-statin treatment interventions (BA+EZE FDC vs EZE) on CV health outcomes such as MACE avoided. Similar modeling approaches have been used previously to estimate cost effectiveness using efficacy data from phase III trials and CV event reduction from CTTC meta-analyses [35]. Simulation models are well-accepted tools that provide a framework for combining the best available evidence to quantify the value of a treatment of interest and can assist clinicians and policy makers in making informed treatment decisions [19, 20, 36].
Results from previous analyses have established that the starting level of risk is often a key driver of model outcomes: the higher a patient’s risk, the more room for benefit [37]. This simulation model-based analysis, therefore, quantifies potential benefits from LDL-C lowering at various baseline levels, instead of a single mean that is not representative of every patient in clinical practice. The results demonstrate that use of BA+EZE FDC in patients not at their LDL-C goal would likely lead to fewer MACE compared with use of EZE alone over a lifetime horizon. Among patients requiring up to a 40% reduction in LDL-C, BA+EZE FDC is predicted to provide a greater risk reduction than EZE alone across all patients and avoids the largest incremental number of CV events relative to EZE among those with a starting LDL-C between 110 and 140 mg/dL. Our findings support the conclusions of an independent advisory panel recommending the use of BA+EZE FDC for patients unlikely to reach LDL-C goals with the addition of EZE alone [38].
Our approach likely underestimates the health outcome benefits of BA+EZE FDC compared with EZE. Efficacy estimates for EZE in this analysis were derived from the EZE arm of the BA+EZE FDC pivotal, phase III registration trial (n = 86; reduction of 25% in LDL-C) [26]. This study represents the only clinical trial data source for which a LDL-C reduction exists for both comparators within the same patient population, as BA+EZE FDC is fairly new to the market. Published meta-analyses that pool findings across multiple studies and other models of EZE [39,40,41] report lower efficacy than the one utilized in this model (range: − 18.6 to − 23.6%); our conservative estimate from this single trial thereby potentially overestimates the benefits of the EZE comparator arm in this analysis.
Recent data from a large observational registry showed that among patients with ASCVD, only 17% had their lipid-lowering therapy intensified after 2 years, and two-thirds remained at LDL-C levels ≥70 mg/dL, despite guidelines recommending the addition of non-statin treatment for patients with LDL-C ≥70 mg/dL who are taking maximally tolerated statins [42]. Such delays or inaction in appropriately managing LDL-C levels have been shown to have detrimental consequences for patients at an increased risk for CV events. In a retrospective cohort study, findings revealed that delays in access to PCSK9 inhibitors led to a higher rate of CV events (7.29 per 100 patient-years) compared with an overall rate of 6.73 per 100 patient-years [43]. In another large retrospective study of patients with ASCVD, findings showed that delayed PCSK9 inhibitor treatment led to a significantly increased risk of CV events (adjusted hazard ratio for composite CV event outcome: 1.11; 95% confidence interval, 1.02–1.22; p = 0.03) compared with those patients who did not experience such a delay [44]. The results of these studies highlight the importance of prescribing the appropriate non-statin treatment to high-risk patients who have not reached their LDL-C goal and are taking maximally tolerated statins.
Baseline LDL-C and CV event risk as well as the potential event reductions through further LDL-C lowering should be considered to optimize the use of non-statin treatments and realize expected long-term benefits in clinical outcomes. This analysis quantifies the incremental benefit of treatment with BA+EZE FDC, a recently approved oral non-statin therapy that offers patients and providers another therapeutic option to help reach LDL-C goals. A simulation analysis published by Blaum et al. evaluated the impact of incorporating BA into lipid-lowering therapy intensification treatment algorithms prior to treatment with a PCSK9 inhibitor, and concluded that the reduction in the downstream need for PCSK9 inhibitors could result in potential cost savings and be particularly favorable for patients not able to tolerate statins [36]. While the impact of BA+EZE FDC was not studied in the simulation by Blaum et al., it should be considered when trying to shorten the time needed to reach recommended LDL-C goals.
In our model, incremental benefits for BA+EZE FDC compared with EZE vary as a function of 10-year risk based on baseline LDL-C in a population of patients with ASCVD and the corresponding number of patients in each subgroup, with an estimated total of 5700 MACE avoided per 100,000 persons across the entire cohort. As the 10-year risk increases, driven by baseline LDL-C, the incremental benefits in MACE avoidance also increase.
4.1 Limitations
This analysis has several limitations. A simulation model relevant to the US setting was used to predict a lifetime risk of CV events in patients with ASCVD rather than observing CV events in a study population. However, it is worthwhile to note that a long-term CV outcomes trial for BA is currently ongoing [45]. Lifetime benefits were also simulated based on an LDL-C reduction at 12 weeks, which was the primary endpoint of the phase III trial. Results from previous studies have demonstrated that improvements in lipid parameters remain consistent for at least 52 weeks after BA treatment [46]. The treatment effects of BA+EZE FDC and EZE were obtained from a small sample of patients (n = 86 in each cohort, respectively) from the phase III clinical trial that was the basis for the US Food and Drug Administration approval [26]. As such, treatment effects may vary in a larger trial or in real-world settings, and thus may reflect an over-estimation or under-estimation of LDL-C lowering of BA+EZE FDC.
The model utilizes the CTTC hazard ratio for stroke, which includes both fatal and nonfatal events, whereas we only examined nonfatal strokes (fatal strokes were incorporated in the “CV death” endpoint). As a result, the model may underestimate the benefit on nonfatal strokes as the relative risk for fatal strokes in CTTC is weaker than that for overall stroke [13]. Similarly, because non-fatal MI is not reported separately, the model may also underestimate its effects as it applies the CTTC hazard ratio for major coronary events for non-fatal MI [13]. In addition, the model uses different data sources to populate all its inputs. We obtained contemporary estimates of MACE from the FOURIER trial placebo arm, which comprised exclusively patients with ASCVD; however, the efficacy estimates for the comparator arms came from a phase III trial comprising patients with ASCVD and/or heterozygous familial hypercholesterolemia (62.4%) and patients with multiple CVD risk factors (37.5%). Although baseline risk estimates could vary across these populations, this is unlikely to be the case as LDL-C lowering has been demonstrated to be consistent across these populations. Further, the use of FOURIER provided base inputs for the model to estimate the cumulative incidence of MACE events. The use of an alternative population would impact the cumulative MACE incidence overall and not necessarily the incremental change between therapies, which was the focus of this analysis.
Finally, this simulation model only focuses on the benefits of LDL-C reductions. While LDL-C is a primary driver of risk and the primary source of risk reduction with these therapies, there are additional sources of risk that are not accounted for in this model. For example, any additional impact of the treatments on other important biomarkers that have previously been demonstrated for BA and BA+EZE FDC (e.g., decreases in high-sensitivity C-reactive protein) are not included in the analysis [26].
5 Conclusions
For the nearly 80% of patients in the USA with ASCVD who are taking maximally tolerated statin doses and have not attained their LDL-C goal, aggressive and prompt addition of non-statin treatment is imperative to avoid CV events. Clinicians should consider current LDL-C levels, CV risk, other patient factors, and access to medications when selecting an appropriate non-statin treatment. In this simulation, addition of BA+EZE FDC to maximally tolerated statins in patients not at their LDL-C goal resulted in more MACE avoided compared with EZE alone over a lifetime horizon.
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics: 2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e28. https://doi.org/10.1161/cir.0000000000000659.
World Health Organization. Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 15 Feb 2019.
American Heart Association. Cardiovascular disease: a costly burden for America. Projections through 2035. 2017. https://www.heart.org/-/media/files/get-involved/advocacy/burden-report-consumer-report.pdf. Accessed 15 Feb 2019.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–143. https://doi.org/10.1161/CIR.0000000000000625.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. https://doi.org/10.1016/s0140-6736(10)61350-5.
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72. https://doi.org/10.1093/eurheartj/ehx144.
Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–97. https://doi.org/10.1001/jama.2016.13985.
Wong ND, Young D, Zhao Y, Nguyen H, Caballes J, Khan I, et al. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;10:1109–18. https://doi.org/10.1016/j.jacl.2016.06.011.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;23:1–6. https://doi.org/10.1177/2047487316653709.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S1-45. https://doi.org/10.1161/01.cir.0000437738.63853.7a.
Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. https://doi.org/10.1016/s0140-6736(14)61368-4.
Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Cholesterol Treatment Trialists’ Collaborators (CTTC), et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. https://doi.org/10.1016/S0140-6736(12)60367-5.
Collaboration CTT. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–15. https://doi.org/10.1016/S0140-6736(18)31942-1.
Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of cholesterol treatment eligibility and medication use among adults: United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2015;64:1305–11. https://doi.org/10.15585/mmwr.mm6447a1.
Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40:243–54. https://doi.org/10.1002/clc.22713.
Handelsman Y, Jellinger PS, Guerin CK, Bloomgarden ZT, Brinton EA, Budoff MJ, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm: 2020 executive summary. Endocr Pract. 2020;26:1196–224. https://doi.org/10.4158/CS-2020-0490.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–350. https://doi.org/10.1016/j.jacc.2018.11.003.
Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices-Modeling Studies. Value Health. 2003;6:9–17. https://doi.org/10.1046/j.1524-4733.2003.00234.x.
Stahl JE. Modelling methods for pharmacoeconomics and health technology assessment: an overview and guide. Pharmacoeconomics. 2008;26:131–48. https://doi.org/10.2165/00019053-200826020-00004.
Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:959–66. https://doi.org/10.1001/jamacardio.2017.2289.
Cannon CP, Khan I, Klimchak AC, Sanchez RJ, Sasiela WJ, Massaro JM, et al. Simulation of impact on cardiovascular events due to lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. Am Heart J. 2019;216:30–41. https://doi.org/10.1016/j.ahj.2019.06.005.
Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39:313–20. https://doi.org/10.1002/clc.22535.
Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–53. https://doi.org/10.1001/jama.2016.11004.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22. https://doi.org/10.1056/NEJMoa1615664.
Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27:593–603. https://doi.org/10.1177/2047487319864671.
Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2013-2014. https://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed 13 Oct 2022.
Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–32. https://doi.org/10.1056/NEJMoa1803917.
Alahmar AE, Nelson CP, Snell KI, Yuyun MF, Musameh MD, Timmis A, et al. Resuscitated cardiac arrest and prognosis following myocardial infarction. Heart. 2014;100:1125–32. https://doi.org/10.1136/heartjnl-2014-305696.
Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10:374–9. https://doi.org/10.1016/j.cardfail.2004.02.003.
Arias E, Heron M, Xu J. United States life tables, 2012. Natl Vital Stat Rep. 2016;65:1–65.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. https://doi.org/10.1161/circulationaha.107.699579.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. https://doi.org/10.1161/01.cir.97.18.1837.
Wilson PW, D’Agostino R Sr, Bhatt DL, Eagle K, Pencina MJ, Smith SC, et al. An international model to predict recurrent cardiovascular disease. Am J Med. 2012;125:695-703.e1. https://doi.org/10.1016/j.amjmed.2012.01.014.
Fonarow GC, Keech AC, Pedersen TR, Giugliano RP, Sever PS, Lindgren P, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069–78. https://doi.org/10.1001/jamacardio.2017.2762.
Blaum C, Brunner FJ, Gossling A, Kroger F, Bay B, Lorenz T, et al. Target populations and treatment cost for bempedoic acid and PCSK9 Inhibitors: a simulation study in a contemporary CAD cohort. Clin Ther. 2021;43:1583–600. https://doi.org/10.1016/j.clinthera.2021.07.019.
Annemans L, Packard CJ, Briggs A, Ray KK. “Highest risk-highest benefit” strategy: a pragmatic, cost-effective approach to targeting use of PCSK9 inhibitor therapies. Eur Heart J. 2018;39:2546–50. https://doi.org/10.1093/eurheartj/ehx710.
Agboola F, Lin GA, Kazi DS, McKenna A, Pearson SD. The effectiveness and value of bempedoic acid and inclisiran for heterozygous familial hypercholesterolemia and secondary prevention of ASCVD. J Manag Care Spec Pharm. 2021;27:961–6. https://doi.org/10.18553/jmcp.2021.27.7.961.
Pandor A, Ara RM, Tumur I, Wilkinson AJ, Paisley S, Duenas A, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265:568–80. https://doi.org/10.1111/j.1365-2796.2008.02062.x.
Zhao Z, Du S, Shen S, Luo P, Ding S, Wang G, et al. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: a frequentist network meta-analysis. Medicine (Baltimore). 2019;98: e14400. https://doi.org/10.1097/md.0000000000014400.
Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii. https://doi.org/10.3310/hta12210 (xi–xiii, 1–212).
Cannon CP, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Gao Q, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021. https://doi.org/10.1001/jamacardio.2021.1810.
Baum SJ, Chen C-C, Rane P, Patel J, Maya J, Harrison D, et al. Cardiovascular risk in patients denied access to PVCK9i therapy. Presentation #1129-408. J Am Coll Cardiol. 2018;71:1760. https://doi.org/10.1016/S0735-1097(18)32301-5.
Myers KD, Farboodi N, Mwamburi M, Howard W, Staszak D, Gidding S, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12: e005404. https://doi.org/10.1161/circoutcomes.118.005404.
Nicholls S, Lincoff AM, Bays HE, Cho L, Grobbee DE, Kastelein JJ, et al. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104–12. https://doi.org/10.1016/j.ahj.2020.10.060.
Ballantyne CM, Banach M, Bays HE, Catapano AL, Laufs U, Stroes ESG, et al. Long-term safety and efficacy of bempedoic acid in patients at high risk of atherosclerotic cardiovascular disease: results from the CLEAR Harmony open-label extension study. Eur Heart J. 2020. https://doi.org/10.1093/ehjci/ehaa946.3344.
Allen JM, Arnold SV, Lohr NL, et al. Abstract 12904: assessing low-density lipoprotein cholesterol risk in secondary prevention patients within the PINNACLE National Outpatient Registry. Circulation. 2019;140:A12904.
Cannon CP, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6:1–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by Esperion Therapeutics, Inc., the manufacturer of bempedoic acid and the fixed-dose combination of bempedoic acid plus ezetimibe used in this study.
Conflicts of Interest/Competing Interests
R. Brett McQueen has received institutional funding to the University of Colorado from Real Endpoints, LLC. Seth J. Baum has received funding and/or consulting fees from Amgen, AstraZeneca, Axcella, Boehringer Ingelheim, Esperion, Lilly, Madrigal, and Novartis. Michael J. Louie is an employee of Esperion Therapeutics, Inc., and may own Esperion stock or stock options. William J. Sasiela is a former (retired) employee of and a current consultant for Esperion Therapeutics, Inc., and may own Esperion stock or stock options. Aikaterini Bilitou is an employee of Daiichi Sankyo Europe, GmbH, which has a corporate agreement with Esperion Therapeutics, Inc. Hemal Shah is an employee of Value Matters, LLC, and a consultant for Esperion Therapeutics, Inc. Beth Nash is a former employee and consultant of Real Endpoints, which received financial support for this study from Esperion Therapeutics, Inc. Kristin K. Gillard is an employee of Esperion Therapeutics and may own Esperion stock or stock options. Kausik K. Ray has received research grants from Amgen, Daiichi Sankyo, MSD, Pfizer, Regeneron, and Sanofi, and honoraria or consulting fees from Abbvie, Algorithm, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cerenis, Cipla, Esperion, IONIS, Kowa, Lilly, Medicines Company, MSD, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Resverlogix, Sanofi, Silence Therapeutics, and Takeda.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data and model that produced the results are proprietary to Esperion Therapeutics, Inc.
Code Availability
Not applicable.
Authors’ Contributions
All authors meet the International Committee of Medical Journal Editors criteria for authorship for this article. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All authors designed the study, RBM conducted the analyses, and BN, HS, KKG, and RBM drafted the manuscript. All authors critically reviewed the manuscript for intellectual content and approved the final version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McQueen, R.B., Baum, S.J., Louie, M.J. et al. Potential Cardiovascular Events Avoided with Bempedoic Acid Plus Ezetimibe Fixed-Dose Combination Compared with Ezetimibe Alone in Patients with Atherosclerotic Cardiovascular Disease Taking Maximally Tolerated Statins. Am J Cardiovasc Drugs 23, 67–76 (2023). https://doi.org/10.1007/s40256-022-00552-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00552-7