Abstract
Background
For patients taking factor Xa (FXa) inhibitors who have life-threatening bleeding, emergency surgery, drug interactions, etc., a rapid and precise assay is needed to monitor for potential medication failure, to assess safety during periprocedural anticoagulation management, and to manage the care of chronically anticoagulated patients. Anti-factor Xa (anti-Xa) activity assays have been recommended in guidelines, but the evaluation of different calibrations of anti-Xa activity assays and the data on the recommended range are still limited, especially in the Asian population.
Methods
This is a nationwide multicenter methodology exploratory study in an Asian population, including nine hospitals from Beijing, Shanghai, Liaoning, Shandong, Jiangsu, Anhui, Henan, Chongqing, and Fujian. A total of 485 healthy volunteers and 219 patients taking rivaroxaban or apixaban (single dose) were enrolled in the study. High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS) was employed to detect plasma rivaroxaban and apixaban. The prothrombin time (PT), activated partial thromboplastin time (APTT), and levels of anti-Xa activity were tested as pharmacodynamic parameters in plasma samples. We evaluated the correlation of anti-Xa activity and blood concentration via HPLC-MS, and then compared the two methods of target drug-calibrated and low-molecular-weight heparin (LMWH)-antithrombin–calibrated anti-Xa activity. Correlations between variables were examined using Pearson’s correlation analysis. Logistic regression was applied to evaluate significant differences in anti-Xa activity and blood concentration, using models adjusted by baseline characteristics.
Results
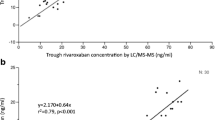
The results suggested anti-Xa activity had better correlation with blood concentrations of apixaban and rivaroxaban than APTT and PT (p < 0.001). Target drug-calibrated anti-Xa activity had better correlation with HPLC-MS results at every dose level and blood collection time (p < 0.001). The expected concentrations (ng/mL) derived from rivaroxaban-calibrated assays of rivaroxaban 10 mg, 15 mg, and 20 mg were about 210, 330, and 270 at peak concentrations, and 28, 44, and 58, respectively, at the trough concentrations.
Conclusions
In this study, we confirm that target drug calibration of anti-Xa activity is a better quantitative detection method for oral direct FXa inhibitors than LMWH-calibrated anti-Xa activity in clinical practice, and expected peak–trough levels are recommended for the Asian population.


Similar content being viewed by others
References
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Martin K, Moll S. Direct oral anticoagulant drug level testing in clinical practice: a single institution experience. Thromb Res. 2016;143:40–4.
Lindhoff-Last E, Samama MM, Ortel TL, et al. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32(6):673–9.
Douxfils J, Chatelain C, Chatelain B, et al. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110(2):283–94.
Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385(9984):2288–95.
Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, Guillermo C, Kawai Y, Lindhoff-Last E, Kitchen S. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–50.
Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
Gehrie E, Laposata M. Test of the month: the chromogenic antifactor Xa assay. Am J Hematol. 2012;84:194–6.
Rathbun S, Tafur A, Grant R, et al. Comparison of methods to determine rivaroxaban anti-factor Xa activity. Thromb Res. 2015;135(2):394–7.
Cuker A, Siegal DM, Crowther MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64(11):1128–39.
Favaloro EJ, Bonar R, Butler J, et al. Laboratory testing for the new oral anticoagulants: a review of current practice. Pathology. 2013;45:435–7.
Gouin-Thibault I, Freyburger G, de Maistre E, et al. Evaluation of dabigatran, rivaroxaban and apixaban target-specific assays in a multicenter French study. Thromb Res. 2017;158:126–33.
Gosselin RC, Francart SJ, Hawes EM, et al. Heparin-calibrated chromogenic anti-Xa activity measurements in patients receiving rivaroxaban: can this test be used to quantify drug level? Ann Pharmacother. 2015;49(7):777–83.
Bookstaver DA, Sparks K, Pybus BS, et al. Comparison of anti-Xa activity in patients receiving apixaban or rivaroxaban. Ann Pharmacother. 2018;52(3):251–6.
Siriez R, Evrard J, Dogné JM, et al. Development of new methodologies for the chromogenic estimation of betrixaban concentrations in plasma. Int J Lab Hematol. 2019;41(2):250–61.
Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16(2):209–19.
Billoir P, Barbay V, Joly LM, et al. Anti-Xa oral anticoagulant plasma concentration assay in real life: rivaroxaban and apixaban quantification in emergency with LMWH calibrator. Ann Pharmacother. 2019;53(4):341–7.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93.
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104.
Douxfils J, Chatelain C, Chatelain B, et al. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110:283–94.
Samoš M, Bolek T, Stančiaková L, et al. Anti-Xa activity in oral factor Xa inhibitor-treated patients with atrial fibrillation and a higher risk of bleeding: a pilot study. Blood Coagul Fibrinolysis. 2018;29(4):369–73.
Cuker A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis. 2016;41(2):241–7.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by grants from the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” of China (No. 2018ZX09201014, No. 2017ZX09101001 and No. 2017ZX09304028), National Natural Science Foundation of China (No. 81872940 and No. 81973395), and Natural Science Foundation of Beijing Municipality (No. 7171012).
Conflict of interest
We declare no competing interests.
Ethics approval and consent to participate
The protocol was approved by an independent ethics committee and the Institutional Review Board of Peking University First Hospital and all participating research sub-central hospitals. The trial registration number is NCT03161496. All subjects were enrolled in this study after signing informed consent.
Consent for publication
The manuscript has been approved by all authors for publication. The work described is original research that has not been published previously; nor is it under consideration for publication elsewhere, in whole or in part.
Availability of data and material
All data related to the study have been shown in the article, and other relevant data can be requested from the authors.
Code availability
Not applicable.
Author contributions
(1) Conception and design: YC, QX, and JJ; (2) provision of study materials or patients: ZL, QX, QX, HZ, GM, and SZ; (3) collection and assembly of data: ZL, QX, QX, HZ, GM, and SZ; (4) data analysis and interpretation: ZL, QX, HZ, and QX; (5) manuscript writing and final approval of manuscript: all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Z., Xie, Q., Zhang, H. et al. Target Drug-Calibrated Anti-Xa Activity Assays and Expected Peak–Trough Levels in an Asian Population: A Multicenter Study. Am J Cardiovasc Drugs 21, 669–679 (2021). https://doi.org/10.1007/s40256-021-00479-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00479-5