Abstract
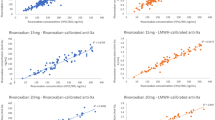
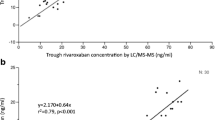
Monitoring of anti-coagulation with the direct factor Xa inhibitor rivaroxaban is considered unnecessary in a routine clinical setting. However, assessment of its anti-coagulant effect may be desirable in certain clinical situations. We assessed prothrombin time (PT) reagents and commercially available anti-Xa assays (Biophen) calibrated for rivaroxaban and heparin in comparison to liquid chromatography–mass spectrometry (LC-MS/MS) measurements of rivaroxaban concentration in samples from patients on treatment with rivaroxaban for stroke prevention in atrial fibrillation. Citrate plasma samples were obtained from 30 randomly selected patients on uninterrupted treatment with rivaroxaban for a minimum of 1 month. The anti-Xa assays, direct Xa inhibitor (DiXa-I®), and Heparin LRT® were conducted for both wide and low calibrations for rivaroxaban. Measurements were compared to LC-MS/MS using correlation, linear regression, intra-class correlation, and Bland–Altman analysis. In 30 patients (9 female) of median age 71.5 years and BMI 26.5 kg/m2, rivaroxaban concentrations between 2.4 and 625 ng/ml (median 82 ng/ml) were measured by LC-MS/MS. PT reagents were poorly correlated with rivaroxaban concentrations (r 2 = 0.52 and 0.09). Anti-Xa assays DiXa-I (r 2 = 0.95) and Heparin LRT (r 2 = 0.97) were correlated with rivaroxaban in all concentrations, but especially in low concentrations with low calibrations (r 2 = 0.97 and 0.98, respectively). The highest agreement occurred between Heparin LRT and low rivaroxaban concentrations with a mean difference of −5.3 ng/ml (limits of agreement, 12.9 to 2.4 ng/ml). Anti-Xa assays can indirectly determine the concentration of rivaroxaban for a wide range of concentrations in real-world patients. An interpretation of anti-Xa and PT measurements in treatment with rivaroxaban requires knowledge of the local reagents.





Similar content being viewed by others
References
Kitchen S, Gray E, Mackie I, Baglin T, Makris M (2014) Measurement of non-Coumarin anticoagulants and their effects on tests of Haemostasis: guidance from the British Committee for Standards in Haematology. Br J Haematol 166:830–841
Cuker A, Siegal DM, Crowther MA, Garcia DA (2014) Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 64:1128–1139
Ten Cate H (2013) New oral anticoagulants: discussion on monitoring and adherence should start now. Thromb J 11:8
Samama MM, Guinet C, Le Flem L (2012) Do new oral anticoagulants require laboratory monitoring? The clinician point of view. Thromb Res 130(Suppl):S88–S89
Rohde G (2008) Determination of rivaroxaban–a novel, oral, direct Factor Xa inhibitor–in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 872:43–50
Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogné J-M, Mullier F (2013) Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost 110:723–731
Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, Le Flem L, Rohde G, Martinoli JL (2012) Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 107:379–387
Francart SJ, Hawes EM, Deal AM, Adcock DM, Gosselin R, Jeanneret C, Friedman KD, Moll S (2014) Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross-sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost 111:1133–1140
Schmitz EM, Boonen K, van den Heuvel DJ, van Dongen JL, Schellings MW, Emmen JM, van der Graaf F, Brunsveld L, van de Kerkhof D (2014) Determination of dabigatran, rivaroxaban and apixaban by UPLC-MS/MS and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J Thromb Haemost 12:1636–1646
Mani H, Rohde G, Stratmann G, Hesse C, Herth N, Schwers S, Perzborn E, Lindhoff-Last E (2012) Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost 108:191–198
Seger C, Tentschert K, Stöggl W, Griesmacher A, Ramsay S (2009) A rapid HPLC-MS/MS method for the simultaneous quantification of cyclosporine A, tacrolimus, sirolimus and everolimus in human blood samples. Nat Protoc 4:526–534
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P (2013) European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 15:625–651
Dale BJ, Ginsberg JS, Johnston M, Hirsh J, Weitz JI, Eikelboom JW (2014) Comparison of the effects of apixaban and rivaroxaban on prothrombin and activated partial thromboplastin times using various reagents. J Thromb Haemost 12:1810–1815
Mani H, Hesse C, Stratmann G, Lindhoff-Last E (2011) Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost 106:156–164
Hillarp A, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Stigendal L, Sten-Linder M, Strandberg K, Lindahl T (2011) Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost 9:133–139
Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE (2010) Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit 32:673–679
Halbmayer W-M, Weigel G, Quehenberger P, Tomasits J, Haushofer AC, Aspoeck G, Loacker L, Schnapka-Koepf M, Goebel G, Griesmacher A (2012) Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 50:1601–1605
Acknowledgments
We thank the clinical collaborators Johannes Thaler, Johanna Gebhart (Department of Hematology and Hemostaseology), Giora Meron (Department of Emergency Medicine/Ophthalmology), Martin Frossard (Department of Emergency Medicine/Trauma Surgery), and Christoph Kratochwill (Department of Internal Medicine II, all Medical University of Vienna) for contributing patient samples. The anti-Xa reagents, calibrators, and controls were kindly provided by CoaChrom Diagnostica GmbH, Maria Enzersdorf, Austria.
Funding
This investigation was supported by an unrestricted grant from Daiichi-Sankyo Austria.
Conflict of interest
The authors declare that they have no conflicts of interest.
Authors contribution
OK acquired data, analyzed and interpreted data, drafted the manuscript, and approved the final version for publication.
SB acquired data, analyzed and interpreted data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
PQ analyzed and interpreted data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
CS designed and validated the LC-MS/MS platform, acquired data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
GW acquired data, critically revised the manuscript for important intellectual content and approved the final version for publication.
AG acquired data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
IP designed and conceived the study interpreted data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
CA designed and conceived the study, analyzed and interpreted data, critically revised the manuscript for important intellectual content and, approved the final version for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Königsbrügge, O., Quehenberger, P., Belik, S. et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol 94, 1463–1471 (2015). https://doi.org/10.1007/s00277-015-2407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2407-y