Abstract
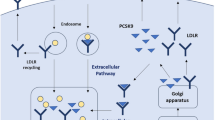
The association between low-density cholesterol (LDL-C) and cardiovascular disease (CVD) is well-established, with an emphasis on lowering LDL-C levels to reduce cardiovascular events. Statin therapy has been the traditional treatment for LDL-C reduction, in addition to lifestyle modifications, but studies have shown that a substantial proportion of patients does not reach target LDL-C goals despite receiving maximally tolerated statin medications. Additionally, statin therapy is associated with a few shortcomings as many patients initiated on these medications discontinue treatment within 1 year because of lack of tolerability. Furthermore, guidelines from both the American College of Cardiology and the American Heart Association highlight the importance of obtaining LDL-C goals because of the residual atherosclerotic CVD risk that remains in high-risk populations. That the residual cardiovascular risk remains despite statin therapy highlights the importance of evaluating therapeutic approaches that possess effective lipid lowering that can be used adjunctively with statins. Much focus has been directed towards the proprotein convertase subtilisin/kexin type 9 (PCSK9) pathway, leading to the development of evolocumab and alirocumab, two human monoclonal antibodies directed against PCSK9. These agents have been shown to markedly decrease LDL-C levels and significantly reduce cardiovascular risk, but the need for biweekly or monthly subcutaneous injections has generated concerns for patient compliance. A new pathway is being studied in which a synthetic small interfering ribonucleic acid (siRNA) targets the PCSK9 gene expressed in hepatocytes to prevent PCSK9 production. The siRNA, inclisiran sodium, significantly reduces hepatic production of PCSK9, causing a marked reduction in LDL-C levels, and exhibits sustained pharmacodynamic effects when dosed subcutaneously every 6 months. This review presents and discusses the current clinical and scientific evidence pertaining to inclisiran sodium.

Similar content being viewed by others
References
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Am Coll Cardiol. 2019;74:177–232.
Ogura MMP. PCSK9 inhibition in the management of familial hypercholesterolemia. J Cardiol. 2018;71:1–7.
Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15:757–69.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–8.
Collaboration CTT. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Perrerault S, Dragomir A, Blais L, et al. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Pharmacoepidemiolo Prescr. 2009;65:1013–24.
Wang N, Fulcher J, Abeysuriya N, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327,037 participants. Lancet Diabetes Endocrinol. 2020;8(1):36–49.
Lin XL, Xiao L, Tang ZH, Jiang ZS, Liu MH. Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed Pharmacother. 2018;104:36–44.
Singh S, Bittner V. Familial hypercholesterolemia—epidemiology, diagnosis, and screening. Curr Atheroscler Rep. 2015;17:1–8.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Gragnano F, Concilio C, Cesaro A, et al. P1513 Adherence to PCSK9 inhibitors in high cardiovascular risk patients in real-world setting: results from a single-center experience and comparison with statin therapy. Eur Heart J. 2017;38(1):314.
Kosmas CE, Silverio D, Ovalle J, Montan PD, Guzman E. Patient adherence, compliance, and perspectives on evolocumab for the management of resistant hypercholesterolemia. Patient Prefer Adherence. 2018;2018(12):2263–6.
Thompson G. Limitations of cholesterol lowering with PCSK9 inhibitors. Lancet Diabetes Endocrinol. 2017;5(4):241–3.
Dyrbus KM, Gasior MM, Penson PM, Ray KKM, Banach MM. Inclisiran—new hope in the management of lipid disorders? J Clin Lipidol. 2020;14:16–27.
Leiter LA, Teoh H, Kallend D, et al. Inclisiran lowers LDL-C and PCSK9 irrespective of diabetes status: the ORION-1 randomized clinical trial. Diabetes Care. 2019;42:173–6.
Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. 2019;4(11):1067–75.
Cupido AJ, Kastelein JJP. Inclisiran for the treatment of hypercholesterolaemia: implications and unanswered questions from the ORION trials. Eur Soc Cardiol. 2020;116:136–9.
Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51.
A Study of Inclisiran in Participants With Renal Impairment Compared to Participants With Normal Renal Function (ORION-7). ClinicalTrials.gov. March 24, 2018. https://clinicaltrials.gov/ct2/show/study/NCT03159416?intr=inclisiran&draw=3&rank=1.
Wright RS, Collins MG, Stoekenbroek RM, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clinc Proc. 2020;95:77–89.
An Extension Trial of Inclisiran Compared to Evolocumab in Participants With Cardiovascular Disease and High Cholesterol (ORION-3). NIH U.S National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03060577. Accessed Oct 2020.
New Long-Term Data Show that Twice-a-Year Dosing with Inclisiran Results in Persistent Lowering of LDL Cholesterol with No Material Safety Observations Out to Three Years. The Medicines Company. May 18, 2019. https://www.themedicinescompany.com/investor/pr/3820269/. Accessed 4 Nov 2020.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30.
European Medicines Agency. Human medicine European public assessment report (EPAR): Leqvio. September 12, 2020. http://www.ema.europa.eu. Accessed 21 Mar 2021.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
Stock J. Targeting LDL cholesterol: early treatment is key to population health. Eur Atheroscler Soc. 2020;300:37–8.
A Study of Inclisiran in Participants With Homozygous Familial Hypercholesterolemia (HoFH) (ORION-5). NIH U.S National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03851705. Accessed Oct 2020.
A randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease (ORION-4). NIH U.S National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03705234. Accessed Oct 2020.
O’Donoghue ML, Fazio S, Guigliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Insights from the FOURIER Trial. Circulation. 2018;139(12):1483–92.
Trial to assess the effect of long term dosing of inclisiran in subjects with high CV risk and elevated LDL-C (ORION-8). NIH U.S National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03814187. Accessed Oct 2020.
The Medicine Company. August 2019. https://www.themedicinescompany.com/media/2019MediaKit_FactSheet_ORION-8_82119.pdf. Accessed 29 Oct 2020.
Liu A. Novartis set to answer inclisiran's FDA rebuff by Q3. But what about an approval timeline? Fierce Pharma. January 26, 2021. https://www.fiercepharma.com. Accessed 21 Mar 2021.
Lui A. Inclisiran now has its cost-effectiveness report from ICER. Should Novartis be concerned? Fierce Pharma. January 29, 2021. https://fiercepharma.com. Accessed 21 Mar 2021.
Lin G, Kazi D, Jih J, Agboola F, Chapman R, Pearson S. Inclisiran and bempedoic acid for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value; evidence report: Institute for Clinical and Economic Review; 2021
Sinning D, Landmesser U. Low-density lipoprotein-cholesterol lowering strategies for prevention of athersclerotic cardiovascular disease: focus on siRNA treatment targeting PCSK9 (Inclisiran). Curr Cardiol Rep. 2020;22(12):1–7.
A study of ALN-PCSSC in participants with homozygous familial hypercholesterolemia (HoFH) - Study results - ClinicalTrials.gov. clinicaltrials.gov.. https://clinicaltrials.gov/ct2/show/results/NCT02963311?term=Inclisiran&draw=2&rank=18. Accessed 23 Apr 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Jennifer Hardy, PharmD; Stephanie Niman, PharmD; Edward Pereira, MD; Todd Lewis, MD; Jessica Reid, PharmD; Rushab Choksi, PharmD; and Rebecca F. Goldfaden, PharmD, have no conflicts of interest that might be relevant to this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors' contributions
All authors contributed to the review. Rebecca Goldfaden had the idea for the article, Stephanie Niman and Jessica Reid performed the literature search and data analysis, and all authors drafted and/or critically revised the review. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Hardy, J., Niman, S., Pereira, E. et al. A Critical Review of the Efficacy and Safety of Inclisiran. Am J Cardiovasc Drugs 21, 629–642 (2021). https://doi.org/10.1007/s40256-021-00477-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00477-7