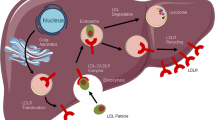
Abstract
Statins remain the mainstay of medical cardiovascular risk reduction because of their effectiveness in decreasing low-density lipoprotein cholesterol (LDL-C) as well as some other potentially beneficial effects. The latest US 2013 lipid guidelines essentially recommend only the prescription of a high-dose statin for the high-risk patient. However, both quite old and quite new outcomes evidence, such as reported for ezetimibe, emphasize that LDL-C lowering is, in and of itself, quite important for cardiovascular risk reduction. It appears that the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors represent a major new contribution to this effort, especially for patients with severe familial hypercholesterolemia, proven clinical cardiovascular disease, statin intolerance, or failure to attain an acceptably low LDL-C goal despite maximum available medical management. Very recent clinical trials have proven overwhelmingly the effectiveness and safety of PCSK9 inhibitors for lowering LDL-C. Both alirocumab and evolocumab have now been approved by the US FDA and there are some initial favorable outcomes data. This review is intended to summarize available evidence and emphasize the possible clinical role of these inhibitors following the approval of alirocumab and evolocumab. Understanding the negative receptor feedback of PCSK9 and the mechanism and beneficial effect of PCSK9 inhibitors for cardiovascular risk reduction is essential for the up-to-date practitioner of cardiovascular medicine. There is every reasonable hope for significant cardiovascular benefit from these new additions to our medical cardiovascular armamentarium.

Similar content being viewed by others
References
Werner C, Hoffmann MM, Winkler K, et al. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vasc Pharmacol. 2014;62(2):94–102.
Norata GD, Tibolla G, Catapano AL. PCSK9 inhibition for the treatment of hypercholesterolemia: promises and emerging challenges. Vasc Pharmacol. 2014;62(2):103–11.
Phan BA, Toth PP. Is the future of statins aligned with new novel lipid modulation therapies? Curr Atheroscler Rep. 2013;15(2):300.
Pisaniello AD, Scherer DJ, Kataoka Y, et al. Ongoing challenges for pharmacotherapy for dyslipidemia. Expert Opin Pharmacother. 2015;16(3):347–56.
Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97.
Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39.
Whayne TF Jr. Guideline confusion for the clinician. Angiology. 2014. doi:10.1177/0003319714559122.
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–504.
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934.
Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1—executive summary. J Clin Lipidol. 2014;8(5):473–88.
European Association for Cardiovascular P, Rehabilitation, Reiner Z, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Jarcho JA, Keaney JF Jr. Proof that lower is better—LDL cholesterol and IMPROVE-IT. N Engl J Med. 2015;372(25):2448–50.
Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol. 2015;66(5):495–507.
Crea F, Niccoli G. Ezetimibe and plaque regression: cholesterol lowering or pleiotropic effects? J Am Coll Cardiol. 2015;66(5):508–10.
Stein EA, Raal FJ. Targeting LDL: is lower better and is it safe? Best Pract Res Clin Endocrinol Metab. 2014;28(3):309–24.
The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364.
The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365-374.
Blankenhorn DH, Johnson RL, Nessim SA, et al. The Cholesterol Lowering Atherosclerosis Study (CLAS): design, methods, and baseline results. Control Clin Trials. 1987;8(4):356–87.
Blankenhorn DH, Nessim SA, Johnson RL, et al. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257(23):3233–40.
Buchwald H, Stoller DK, Campos CT, et al. Partial ileal bypass for hypercholesterolemia. 20- to 26-year follow-up of the first 57 consecutive cases. Ann Surg. 1990;212(3):318–29 (discussion 329–331).
Buchwald H, Varco RL, Boen JR, et al. Effective lipid modification by partial ileal bypass reduced long-term coronary heart disease mortality and morbidity: five-year posttrial follow-up report from the POSCH. Program on the Surgical Control of the Hyperlipidemias. Arch Intern Med. 1998;158(11):1253–61.
Schonfeld G. The hypobetalipoproteinemias. Annu Rev Nutr. 1995;15:23–34.
Cohen JC, Boerwinkle E, Mosley TH Jr, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
Stegman B, Puri R, Cho L, et al. High-intensity statin therapy alters the natural history of diabetic coronary atherosclerosis: insights from SATURN. Diabetes Care. 2014;37(11):3114–20.
Wiviott SD, Cannon CP, Morrow DA, et al. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46(8):1411–6.
Hsia J, MacFadyen JG, Monyak J, et al. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol. 2011;57(16):1666–75.
Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485–94.
Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18(4):220–8.
Stawowy P, Kelle S, Fleck E. PCSK9 as new target in hyperlipidemia treatment. Herz. 2014;39(4):466–9.
Peterson AS, Fong LG, Young SG. PCSK9 function and physiology. J Lipid Res. 2008;49(6):1152–6.
Awan Z, Baass A, Genest J. Proprotein convertase subtilisin/kexin type 9 (PCSK9): lessons learned from patients with hypercholesterolemia. Clin Chem. 2014;60(11):1380–9.
Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. 2007;32(2):71–7.
Leren TP. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet. 2004;65(5):419–22.
Lepor NE, Contreras L, Desai C, et al. The potential role of anti-PCSK9 monoclonal antibodies in the management of hypercholesterolemia. Rev Cardiovasc Med. 2014;15(4):290–307 (quiz 308–299).
Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891–900.
Sahebkar A, Simental-Mendia LE, Guerrero-Romero F, et al. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: a systematic review and meta-analysis of clinical trials. Diabetes Obes Metab. 2015;17(11):1042–48.
Sahebkar A. Circulating levels of proprotein convertase subtilisin kexin type 9 are elevated by fibrate therapy: a systematic review and meta-analysis of clinical trials. Cardiol Rev. 2014;22(6):306–12.
Catapano AL, Papadopoulos N. The safety of therapeutic monoclonal antibodies: implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis. 2013;228(1):18–28.
Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol. 2012;59(25):2354–5.
Costet P, Hoffmann MM, Cariou B, et al. Plasma PCSK9 is increased by fenofibrate and atorvastatin in a non-additive fashion in diabetic patients. Atherosclerosis. 2010;212(1):246–51.
Milionis H, Liamis G, Elisaf M. Proprotein convertase subtilisin kexin 9 inhibitors: next generation in lipid-lowering therapy. Expert Opin Biol Ther. 2015;15(2):287–98.
Li C, Lin L, Zhang W, et al. Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta-analysis of 20 Randomized Controlled Trials. J Am Heart Assoc. 2015;4(6):e001937.
Tavori H, Melone M, Rashid S. Alirocumab: PCSK9 inhibitor for LDL cholesterol reduction. Expert Rev Cardiovasc Ther. 2014;12(10):1137–44.
Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176(1):55–61.
Sanofi and Regeneron present detailed positive results from four pivotal alirocumab trials at ESC Congress 2014. 2014. http://en.sanofi.com/Images/37129_20140831_AlirocumabESCDataRelease_en.pdf. Accessed 20 Oct 2015.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99.
Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–19.
Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–40.
Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–50.
Koren MJ, Giugliano RP, Raal FJ, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129(2):234–43.
Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–40.
Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–82.
Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9.
Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). 2015. https://clinicaltrials.gov/ct2/show/NCT01764633. Accessed 12 Aug 2015.
The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). 2015. https://clinicaltrials.gov/ct2/show/NCT01975389. Accessed 12 Aug 2015.
Carroll J. Alnylam, Medicines Co. say next-gen PCSK9 drug has blockbuster potentia. 2015. http://www.fiercebiotech.com/story/alnylam-medicines-co-say-next-gen-pcsk9-drug-has-blockbuster-potential/2015-08-29. Accessed 20 Oct 2015.
Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128(9):962–9.
Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63(13):1278–88.
ClevelandClinic. OSLER results offer early encouragement on longer-term safety and efficacy of PCSK9 inhibitor. 2015. http://consultqd.clevelandclinic.org/2015/03/osler-results-offer-early-encouragement-on-longer-term-safety-and-efficacy-of-pcsk9-inhibitor/. Accessed 20 Oct 2015.
Swiger KJ, Martin SS. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf. 2015;38(6):519–26.
Postmus I, Trompet S, de Craen AJ, et al. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res. 2013;54(2):561–6.
Swain ET, A. FDA advisory panel backs approval of PCSK9 inhibitors. Cardiology Today 2015. http://www.healio.com/cardiology/chd-prevention/news/print/cardiology-today/%7Ba48c08c5-0d4d-4cb2-8706-010ee8a95886%7D/fda-advisory-panel-backs-approval-of-pcsk9-inhibitors. Accessed 20 Oct 2015.
Release FN. FDA approves Repatha to treat certain patients with high cholesterol. 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm460082.htm. Accessed 28 Aug 2015.
Drug Analyst Consensus Database: Alirucomab. 2015. http://consensus.druganalyst.com/Guest/Sanofi/Praluent. Accessed 28 July 2015.
Repatha (evolucomab)-First PCSK9 inhibitor in the world is approved in Europe. 2015. http://essentialguidetoprescriptiondrugs.com/article/repatha-evolucomab-first-pcsk9-inhibitor-world-approved-europe. Accessed 28 July 2015.
Ontario HQ. Low-density lipoprotein apheresis: an evidence-based analysis. Ont Health Technol Assess Ser. 2007;7(5):1–101.
Konarzewski M, Szolkiewicz M, Sucajtys-Szulc E, et al. Elevated circulating PCSK-9 concentration in renal failure patients is corrected by renal replacement therapy. Am J Nephrol. 2014;40(2):157–63.
Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, et al. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One. 2014;9(9):e106294.
Khera AV, Qamar A, Reilly MP, et al. Effects of niacin, statin, and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 levels in patients with dyslipidemia. Am J Cardiol. 2015;115(2):178–82.
Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14.
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012–22.
Kastelein JJ, Besseling J, Shah S, et al. Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet. 2015;385(9983):2153–61.
Dezima Reports Positive Results in its Phase 2b TULIP Trial with CETP Inhibitor TA-8995 in Dyslipidemia. 2014. http://www.businesswire.com/news/home/20140829005015/en/Dezima-Reports-Positive-Results-Phase-2b-TULIP. Accessed 12 Aug 2015.
FDA Approves Aegerion Pharmaceuticals’ JUXTAPID(TM) (lomitapide) Capsules for Homozygous Familial Hypercholesterolemia (HoFH). 2012. http://ir.aegerion.com/releasedetail.cfm?ReleaseID=728650. Accessed 12 Aug 2015.
European Primary Care Cardiovascular Society: Another PCSK9-inbitor on its way to European approval for hypercholesterolaemia. 2015. http://www.epccs.eu/d/467/another-pcsk9-inbitor-on-its-way-to-european-approval-for-hypercholesterolaemia. Accessed 11 Aug 2015.
Acknowledgments
The author wishes to acknowledge the excellent editorial critique and contribution of Paula M. Heron, PhD, to this review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The author declares no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Whayne, T.F. Defining the Role of PCSK9 Inhibitors in the Treatment of Hyperlipidemia. Am J Cardiovasc Drugs 16, 83–92 (2016). https://doi.org/10.1007/s40256-015-0150-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-015-0150-3