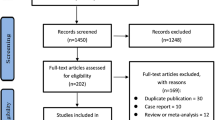
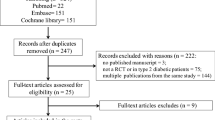
Abstract
Background
The treatment duration of insulin-sodium-glucose co-transporter inhibitors (SGLTis) co-treatment of type 1 diabetes mellitus (T1DM) patients in randomized controlled trials (RCTs) varies by 1–52 weeks. Henceforth, treatment duration-wise, we compared the following insulin-treatment adjuncts- mega- versus low-dose SGLTis, SGLTis versus placebo, and different SGLTi dosages.
Method
Double-blinded RCTs reporting the above were searched (using terms like insulin-dependent, "juvenile-onset diabetes," and “sodium glucose cotransport*") in the PubMed, Embase, and Scopus databases and appraised using a Cochrane tool. The risks across different SGLTi-dosages were compared using network meta-analysis. Random-effect pairwise meta-analysis was performed for the remaining harm juxtapositions. Meta-analyses were performed for the following treatment durations- < 4 weeks, 4 to < 24 weeks, and ≥ 24 weeks. For meta-analysis and certainty of evidence assessment, we used the Stata statistical software and the GRADE method, respectively.
Results
A total of 15 (low risks of bias) studies sourcing data from about 7,330 T1DM patients were reviewed. Meta-analysis findings of ≥ 24 weeks long trials were- a. SGLTi-insulin co-treatment increased the genital infection (GI) (RR: 3.51; 95% CI: 2.59, 4.77), diabetic ketoacidosis (DKA) and (RR: 3.25; 95% CI:1.29, 8.16), and serious side effects (RR: 1.43; 95% CI: 1.05, 1.94) risk. b. SGLT2i-insulin increased the GI risk (RR: 3.77; 95% CI: 2.31, 6.16; high-quality evidence). c. Sotagliflozin-insulin increased the GI (RR: 3.36; 95% CI: 2.28, 4.96) and DKA (RR: 6.69; 95% CI: 2.75, 16.32) risk (both high-quality evidence). Compared to low-dose, megadose SGLTi treatment for 4 to < 24 weeks increased the GI risk. The remaining analyses were not statistically significantly different.
Conclusion
On moderate to long-term treatment (24–52 weeks) of T1DM patients, insulin-SGLT2i co-treatment was associated with GI risk, and insulin-sotagliflozin co-treatment was associated with DKA and GI risk.







Similar content being viewed by others
Data Availability
The full dataset used for the analysis conducted in this paper is available in S4 File.
References
IDF Diabetes Atlas. International Diabetes Federation, 10th edn [Internet]. Brussels, Belgium; 2021 [cited 2022 Nov 2]. Available from: https://www.diabetesatlas.org.
Los E, Wilt AS. Diabetes Mellitus Type 1 In Children [Internet]. StatPearls. 2022. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28722947.
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med [Internet]. 1993;329:977–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8366922.
Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care [Internet]. 2015;38:971–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25998289.
Elhabashy SA, Ezz elarab HS, Thabet RA, Oda AS. Assessment of self-monitoring of blood glucose in type 1 diabetic children and adolescents and its influence on quality of life: practice and perspective. Egypt Pediatr Assoc Gaz [Internet]. 2020;68:22. Available from: https://epag.springeropen.com/articles/10.1186/s43054-020-00028-w.
Thota S, Akbar A. Insulin [Internet]. StatPearls. 2022. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32809523.
Dellepiane S, Ben Nasr M, Assi E, Usuelli V, Letizia T, D’Addio F, et al. Sodium glucose cotransporters inhibitors in type 1 diabetes. Pharmacol Res [Internet]. 2018;133:1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29689314.
Dapagliflozin (Forxiga): no longer authorised for treatment of type 1 diabetes mellitus - GOV.UK [Internet]. [cited 2022 Nov 6]. Available from: https://www.gov.uk/drug-safety-update/dapagliflozin-forxiga-no-longer-authorised-for-treatment-of-type-1-diabetes-mellitus.
Zynquista | European Medicines Agency [Internet]. [cited 2022 Nov 6]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zynquista#authorisation-details-section.
Overview | Sotagliflozin with insulin for treating type 1 diabetes | Guidance | NICE [Internet]. [cited 2022 Nov 6]. Available from: https://www.nice.org.uk/guidance/ta622.
Press Release Approval of Suglat ® Tablets, Selective SGLT2 Inhibitor, for Additional Indication of type 1 diabetes mellitus and Additional dosage and administration in Japan [Internet]. 2018 [cited 2022 Nov 6]. Available from: https://www.astellas.com/en/system/files/news/2018-12/181221_2_Eg_2.pdf.
Markham A, Keam SJ. Sotagliflozin: First global approval. Drugs [Internet]. 2019;79:1023–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31172412.
Wolfsdorf JI, Ratner RE. SGLT inhibitors for type 1 diabetes: Proceed with extreme caution. Diabetes Care [Internet]. 2019;42:991–3. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dci19-0008.
U.S. Food and Drug Administration. Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors [Internet]. 2018 [cited 2022 Nov 2]. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/sodium-glucose-cotransporter-2-sglt2-inhibitors.
Watada H, Shiramoto M, Ueda S, Tang W, Asano M, Thorén F, et al. Pharmacokinetics and pharmacodynamics of dapagliflozin in combination with insulin in Japanese patients with type 1 diabetes. Diabetes, Obes Metab [Internet]. 2019;21:876–82. Available from: http://doi.wiley.com/10.1111/dom.13593.
Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, et al. HbA 1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: The European inTandem2 study. Diabetes Care [Internet]. 2018;41:1981–90. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc18-0342.
Saha S, Saha S. A systematic review and meta-analysis of randomized controlled trials comparing the safety of dapagliflozin in type 1 diabetes patients. Iran J Heal Sci [Internet]. 2020;8. Available from: http://jhs.mazums.ac.ir/article-1-708-en.html.
Yamada T, Shojima N, Noma H, Yamauchi T, Kadowaki T. Sodium‐glucose co‐transporter‐2 inhibitors as add‐on therapy to insulin for type 1 diabetes mellitus: Systematic review and meta‐analysis of randomized controlled trials. Diabetes, Obes Metab [Internet]. 2018;20:1755–61. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.13260.
Saha S. A juxtaposition of safety outcomes between various doses of sodium-glucose co-transporter inhibitors, in insulin-treated type-1 diabetes mellitus patients: a protocol for systematic review and meta-analysis of randomized controlled trials. J Ideas Heal [Internet]. 2020;3:167–72. Available from: http://www.jidhealth.com/index.php/jidhealth/article/view/56.
Saha S, Saha S. Comparison between sodium-glucose co-transporter inhibitors of high dose and low dose, and with placebo in contemporary insulin-treated type-1 diabetes patients: a systematic review and meta-analysis of randomised controlled trials. [Internet]. PROSPERO 2019 CRD42019146578. [cited 2019 Dec 8]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019146578.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med [Internet]. 2015;162:777–84. Available from: https://www.acpjournals.org/doi/abs/10.7326/M14-2385.
Saha S, Saha S, Gayen M. IDF21–0150 A dose-based comparison of safety among sodium-glucose co-transporter inhibitor-treated type1 diabetes patients. Diabetes Res Clin Pract [Internet]. 2022;186:109476. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168822722002881.
Ioannidis JPA. Better reporting of harms in randomized trials: An extension of the CONSORT statement. Ann Intern Med [Internet]. 2004;141:781. Available from: http://annals.org/article.aspx?doi=10.7326/0003-4819-141-10-200411160-00009.
Chernecky C, Berger B. Laboratory tests and diagnostic procedures 6th Edition [Internet]. 6th ed. Elev. Saundres, St. Louis. Elsevier; 2013 [cited 2021 Dec 11]. Available from: https://www.elsevier.com/books/laboratory-tests-and-diagnostic-procedures/chernecky/978-1-4557-0694-5.
Musso G, Gambino R, Cassader M, Paschetta E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ [Internet]. 2019;365:l1328. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30967375.
Saha S, Saha S. A systematic review and meta-analysis of randomised controlled trials, contrasting the safety profile between sodium-glucose cotransporter-2 inhibitors and placebo in type 1 diabetes mellitus patients. Int J Diabetes Metab [Internet]. S. Karger AG; 2019;25:62–73. Available from: https://www.karger.com/Article/FullText/506366.
Saha S, Saha S. Efficacy and safety of empagliflozin in type 1 diabetes mellitus patients: A systematic review. Turkish J Endocrinol Metab [Internet]. 2021 [cited 2021 Nov 23];25:426–38. Available from: http://www.turkjem.org/current-articleinpress/efficacy-and-safety-of-empagliflozin-in-type-1-diabetes-mellitus-patients-a-systematic-review-15035.
Saha S, Saha S. The comparison of efficacy and safety between different doses of empagliflozin in insulin-treated type 1 diabetes mellitus patients: a systematic review and meta-analysis protocol. J Diabetes Metab Disord [Internet]. 2020;19:545–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32550206.
Saha S, Saha S. A systematic review and meta-analysis of randomized controlled trials, juxtaposing the control of glycemia and blood pressure between large dose empagliflozin and placebo among type 1 diabetes patients. Int J Health Sci (Qassim) [Internet]. 2020;14:40–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32206059.
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane Handb Syst Rev Interv [Internet]. Wiley; 2019. p. 205–28. Available from: https://onlinelibrary.wiley.com/doi/10.1002/9781119536604.ch8.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ [Internet]. 2003;327:557–60. Available from: http://www.bmj.com/cgi/doi/10.1136/bmj.327.7414.557.
Saha S. Death and invasive mechanical ventilation risk in hospitalized COVID-19 patients treated with anti-SARS-CoV-2 monoclonal antibodies and/or antiviral agents: A systematic review and network meta-analysis protocol. Chen RJ, editor. PLoS One [Internet]. 2022;17:e0270196. Available from: https://dx.plos.org/10.1371/journal.pone.0270196.
Saha S, Saha S. The effects of prenatal dietary supplements on blood glucose and lipid metabolism in gestational diabetes mellitus patients: A systematic review and network meta-analysis protocol of randomized controlled trials. Laganà AS, editor. PLoS One [Internet]. 2022;17:e0267854. Available from: https://dx.plos.org/10.1371/journal.pone.0267854.
Saha S. Comparative effectiveness of adjunct non-pharmacological interventions on maternal and neonatal outcomes in gestational diabetes mellitus patients: A systematic review and network meta-analysis protocol of randomized controlled trials. Grammatikopoulou MG, editor. PLoS One [Internet]. 2022;17:e0263336. Available from: https://dx.plos.org/10.1371/journal.pone.0263336.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods [Internet]. 2006;11:193–206. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16784338.
Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). [Internet]. Cochrane Handb. Syst. Rev. Interv. version 6.2 (updated Febr. 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook.
Song F, Altman DG, Glenny A-M, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ [Internet]. 2003;326:472. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12609941.
Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess [Internet]. 2005;9:1–134, iii–iv. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16014203.
Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS One [Internet]. 2010;5:e11054. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21085712.
Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods [Internet]. 2012;3:80–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26062083.
Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev [Internet]. 2017;6:79. Available from: http://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-017-0473-z.
Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. Haibe-Kains B, editor. PLoS One [Internet]. 2013;8:e76654. Available from: https://dx.plos.org/10.1371/journal.pone.0076654.
Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods [Internet]. 2012;3:161–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26062088.
Schünemann H, Brożek J, Guyatt G, Oxman A, Editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. GRADE Work Group, 2013 [Internet]. 2013; Available from: guidelinedevelopment.org/handbook.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLOS Med [Internet]. 2020;17:e1003082. Available from: https://dx.plos.org/10.1371/journal.pmed.1003082.
Boeder S, Edelman SV. Sodium‐glucose co‐transporter inhibitors as adjunctive treatment to insulin in type 1 diabetes: A review of randomized controlled trials. Diabetes, Obes Metab [Internet]. 2019;21:62–77. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.13749.
Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med [Internet]. 2017;377:2337–48. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1708337.
Baker C, Wason S, Banks P, Sawhney S, Chang A, Danne T, et al. Dose‐dependent glycometabolic effects of sotagliflozin on type 1 diabetes over 12 weeks: The inTandem4 trial. Diabetes, Obes Metab [Internet]. 2019;21:2440–9. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.13825.
Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris A-G, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: A randomized, double-blind, placebo-controlled pilot study. Diabetes Care [Internet]. 2015;38:412–9. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc13-2955.
Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. lancet Diabetes Endocrinol [Internet]. 2017;5:864–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28919061.
Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: The EASE trials. Diabetes Care [Internet]. 2018;41:2560–9. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc18-1749.
Shimada A, Hanafusa T, Yasui A, Lee G, Taneda Y, Sarashina A, et al. Empagliflozin as adjunct to insulin in Japanese participants with type 1 diabetes: Results of a 4-week, double-blind, randomized, placebo-controlled phase 2 trial. Diabetes, Obes Metab [Internet]. 2018;20:2190–9. Available from: http://doi.wiley.com/10.1111/dom.13351.
Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: The North American inTandem1 study. Diabetes Care [Internet]. 2018;41:1970–80. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc18-0343.
Kaku K, Isaka H, Toyoshima J, Sakatani T. Clinical pharmacology study of ipragliflozin in Japanese patients with type 1 diabetes mellitus: A phase 2, randomized, placebo‐controlled trial. Diabetes, Obes Metab [Internet]. 2019;21:1445–54. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.13679.
Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care [Internet]. 2018;41:1938–46. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc18-0623.
Pieber TR, Famulla S, Eilbracht J, Cescutti J, Soleymanlou N, Johansen OE, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes, Obes Metab [Internet]. 2015;17:928–35. Available from: http://doi.wiley.com/10.1111/dom.12494.
Bode BW, Cengiz E, Wadwa RP, Banks P, Danne T, Kushner JA, et al. Effects of sotagliflozin combined with intensive insulin therapy in young adults with poorly controlled type 1 diabetes: The JDRF sotagliflozin study. Diabetes Technol Ther [Internet]. 2021;23:59–69. Available from: https://www.liebertpub.com/doi/10.1089/dia.2020.0079.
Kaku K, Isaka H, Sakatani T, Toyoshima J. Efficacy and safety of ipragliflozin add‐on therapy to insulin in Japanese patients with type 1 diabetes mellitus: A randomized, double‐blind, phase 3 trial. Diabetes, Obes Metab [Internet]. 2019;21:2284–93. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.13807.
Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care [Internet]. 2015;38:1181–8. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc14-2806.
Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium–glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care [Internet]. 2015;38:2258–65. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc15-1730.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ [Internet]. 2021;n71. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.n71.
Melander H, Ahlqvist-Rastad J, Meijer G, Beermann B. Evidence b(i)ased medicine--selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications. BMJ [Internet]. 2003;326:1171–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12775615.
Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ [Internet]. 2003;326:1167–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12775614.
Yang Y, Chen S, Pan H, Zou Y, Wang B, Wang G, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes. Medicine (Baltimore) [Internet]. 2017;96:e6944. Available from: http://insights.ovid.com/crossref?an=00005792-201705260-00027.
Saha S. An appraisal of a systematic review and meta-analysis of randomized clinical trials on the efficacy and safety of sodium-glucose cotransporter-2 inhibitors as an adjunct to insulin therapy in type 1 diabetes patients. Int J Diabetes Metab [Internet]. S. Karger AG; 2019;25:162–162. Available from: https://www.karger.com/Article/FullText/502743.
El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract [Internet]. 2018;137:83–92. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016882271731344X.
Chen J, Fan F, Wang JY, Long Y, Gao CL, Stanton RC, et al. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis. Sci Rep [Internet]. 2017;7:44128. Available from: http://www.nature.com/articles/srep44128.
Kuhadiya ND, Ghanim H, Mehta A, Garg M, Khan S, Hejna J, et al. Dapagliflozin as additional treatment to liraglutide and insulin in patients with type 1 diabetes. J Clin Endocrinol Metab [Internet]. 2016;101:3506–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27490915.
Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev [Internet]. 2017;2017. Available from: http://doi.wiley.com/10.1002/14651858.MR000033.pub3.
Fabbri A, Lai A, Grundy Q, Bero LA. The influence of industry sponsorship on the research agenda: A scoping review. Am J Public Health [Internet]. 2018;108:e9–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30252531.
Author information
Authors and Affiliations
Contributions
SS1 conceptualized, designed, performed the analysis, and prepared the first and final draft of the manuscript. SS1 and SS2 contributed to database search, study selection, and data abstraction. SS1, SS2, and MG contributed to the risk of bias assessment. MG checked abstracted data for errors. All authors read and agreed to the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, S., Saha, S. & Gayen, M. Treatment-duration-wise harm profile of insulin-sodium-glucose co-transporter inhibitor co-treatment in type 1 diabetes mellitus patients. J Diabetes Metab Disord 22, 673–701 (2023). https://doi.org/10.1007/s40200-023-01192-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01192-7