Abstract
Purpose of Review
Complex regional pain syndrome (CRPS) is a debilitating condition that manifests with sensory, neurologic, autonomic, and/or trophic impairment. In addition to manifesting with severe neuropathic pain, CRPS is associated with poor quality of life and higher annual healthcare costs. This systematic review appraises the current body of evidence on all treatment modalities for CRPS.
Recent Findings
In patients with CRPS-related pain, there is level I evidence supporting modest to moderate improvement in pain intensity from physical therapy, occupational therapy, massage therapy, acupuncture, and transcutaneous electrical nerve stimulation (TENS), although changes in functionality were inconsistent. Topical medications such as eutectic mixture of local anesthetic (EMLA) and ketamine cream were associated with decreased allodynia and hyperalgesia. Inconsistency was present in the current literature in terms of the analgesic effects of gabapentinoids for CRPS. Patients who received intramuscular or intravenous bisphosphonate therapy may achieve modest to moderate improvement in pain intensity and functionality. Systemic steroid and ketamine provided only short-term pain reduction. In terms of interventional therapy, there was an association of modest to moderate improvement in pain with sympathetic ganglion block, sympathectomy, dorsal column spinal cord stimulation, dorsal root ganglion stimulation, and peripheral nerve stimulation, although the level of evidence was limited.
Summary
In summary, the purpose of this systematic review is to equip the clinician with important updates on conservative, pharmacologic, and interventional treatment modalities for CRPS-related pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex regional pain syndrome (CRPS) is a debilitating and painful condition characterized by sensory, neurologic, autonomic, and/or trophic impairments [1]. CRPS is a clinical diagnosis based on accepted criteria including the Veldman criteria, the International Association for the Study of Pain (IASP) criteria for CRPS, Valencia criteria, and most commonly the Budapest criteria [2,3,4]. The global prevalence of CRPS is estimated between 5.5 and 26.2 per 100,000 persons per year [5•] affecting more females than males between the ages of 40 and 70 years old [6]. The total annual healthcare costs and prescription costs after a diagnosis of CRPS are estimated to be 2.17-fold and 2.56-fold higher compared to baseline, respectively [7].
The exact pathophysiologic mechanism of CRPS is unknown. It has been postulated that CRPS is a result of a multifactorial derangement in the inflammatory system, immune system, peripheral and central pain signaling pathways, and autonomic nervous system [1, 2]. The primary objective of this scoping review is to describe the available treatment modalities for CRPS. In addition, we appraise the level of evidence and degree of recommendation for each treatment modality, as well as propose an updated treatment algorithm for CRPS.
Methods
Search Strategy and Study Selection
An entry was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database before study initiation. A systematic search was performed by an experienced librarian (L.C.H.) after search terms were previously determined by the study authors. Relevant keywords included the following: “physical therapy,” “acupuncture,” “transcutaneous electrical nerve stimulation,” “amitriptyline,” “nortriptyline,” “ketamine,” “gabapentin,” pregabalin,” “tricyclic antidepressant,” “opioid,””neuromodulation,” “spinal cord stimulation,” “dorsal root ganglion stimulation,” “peripheral nerve stimulation,” “intrathecal systemic steroid,” “scrambler therapy,” “cannabis,” “bisphosphonates,” “topical diclofenac,” “topical lidocaine,” “sympathetic nerve block,” “sympathetic neural lysis,” “surgery,” and “treatment of complex regional pain syndrome.” Databases Ovid MEDLINE(R), Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus were queried from 1990 to the search date (April 26, 2023) for the selected search terms and synonyms. The actual strategies listing all search terms, Boolean operators, and how they were combined are available in eAppendix 1. All articles were screened by two authors (Y.F.H. and E.K.) independently including titles, abstracts, and full texts. Inclusion criteria included observational studies and randomized controlled trials (RCTs) in the English language that reported change in pain intensity after implementation of a treatment modality (conservative, pharmacologic, or interventional) for CRPS. Exclusion criteria included case reports or case series and animal studies. Discrepancies were resolved by a third author (R.S.D.).
Data Extraction
Study characteristics (study design, funding source, treatment modality, and sample size) and outcomes of interest were extracted. Primary outcome was change in pain intensity from baseline. Secondary outcomes included change in physical functioning and mental health. For each treatment modality, RCTs were selected for further data extraction and analysis. If no RCTs were available, observational or retrospective studies were selected.
Evidence Appraisal
We utilized the United States Preventive Services Task Force (USPSTF) Criteria to appraise the evidence level and degree of recommendation for each treatment modality (eAppendix 2 and eAppendix 3). A degree of recommendation A correlates to the highest level of recommendation where there is good evidence that the measure is effective, and benefits outweigh the harms. Conversely, a degree of recommendation D correlates the lowest level of recommendation where there is at least moderate evidence that the measure is ineffective. A degree of recommendation I correlates to insufficient, low-quality, or contradictory evidence and that recommendation cannot be determined.
Results
Search Strategy
Of 3027 studies that were screened after deduplication, 322 studies underwent full-text review (Fig. 1). Of studies that underwent full-text review, sixty-five studies were included: 53 RCTs (9–19, 22–27, 31–56, 60–67, 71, 82, 83, 85, 86), 6 prospective studies (20, 21, 28, 29, 74, 81), and 6 retrospective studies (30, 72, 73, 78–80). The key study characteristics are reported in Table 1, 2, 3, and 4.
Clinical Presentation
History
Patients with a suspected diagnosis of CRPS typically present with pain that is disproportionate to a recent trauma in a non-dermatomal distribution [2]. CRPS typically manifests with abnormalities in sensory, vasomotor, sudomotor, and/or motor/tropic signs and symptoms. The pain may migrate from the site of initial injury towards the torso and even to the contralateral limb [4].
Physical Exam
The physical examination should be conducted by exposing the affected limb and non-affected limb to assess for evidence of sensory, vasomotor, sudomotor/oedema, and motor/trophic abnormalities as listed by the Budapest Criteria [4]. Neurological and vascular exams should be performed to make sure that the presenting symptoms are not explained by a central/peripheral nerve injury or vascular pathology.
Diagnostic Tests
There is no single diagnostic test or lab for CRPS. CRPS is a clinical diagnosis. However, diagnostic workup should be considered in patients presenting with a suspected diagnosis of CRPS to rule out other pathologies. Labs include a complete blood count, C-reactive protein, erythrocyte sedimentation rate, and/or anti-nuclear antibody to rule out infectious, inflammatory, and/or auto-immune pathologies [3, 75]. Nerve conduction tests and electromyography may also be conducted to assess for peripheral nerve lesions. Thermography, sweat test, bone scintigraphy in the acute phase, triple-phase bone scintigraphy within the first 5 months, plain radiographs in the chronic phase, bone mineral density, and sympathetic block may also support a diagnosis of CRPS.
Conservative Treatment Options
This section describes conservative treatments for CRPS including physical therapy (PT), mirror therapy (MT), acupuncture, and transcutaneous electrical stimulation (TENS) (Table 1). Six RCTs comprising 317 participants were identified that investigated the effects of PT on patients with CRPS [8,9,10,11,12,13]. Lee et al. reported that PT reduced pain and improved gait impairment [8]. Adding aerobic exercise to PT was superior to PT alone [9]. Moreover, pain exposure PT resulted in improved disability and function compared to protective pain contingent treatment [10, 11]. Oerlemans et al. demonstrated that PT was superior to occupational therapy (OT) in reducing pain and improving active range of motion [12]. PT was more cost-effective [13]. Overall, there was favorable improvement in pain intensity (level I, degree B) and physical functioning outcomes (level I, degree B) following PT for CRPS.
Three RCTs comprising of 106 patients evaluated the effectiveness of MT on patients with CRPS [14,15,16]. Cacchio et al. showed that MT improved pain and motor function compared to controls [14]. Adding MT to conventional therapy resulted in higher functional independence measure-motor and -pain scores [15]. For the maximal impact on pain and disability, MT needed to be performed in a sequential order such as hand laterality recognition, imagined movements, and mirror movements [16]. Overall, the literature supports the association of MT and improvement in pain intensity (level I, degree B) and physical functioning outcomes (level 1, degree B) in CRPS.
The efficacy of acupuncture in treating CRPS was investigated in two RCTs [17, 18] and two prospective case–control studies [18] which collectively comprised of 372 patients. Korpan et al. observed no statistical difference in pain intensity in CRPS patients treated with acupuncture or sham [17]. However, electric acupuncture was demonstrated to improve pain and functionality [18, 19]. Additionally, Zheng et al. (2018) showed that combining acupuncture with rehabilitation led to improvement in upper extremity motor function and enhancement of quality of life [20]. Overall, the association of acupuncture in improvement of pain intensity (level I, degree C) and functionality (level I, degree C) in CRPS varied among the studies and its benefit remains unclear.
One RCT comprising 30 patients reported on the use of TENS in the management of CRPS [21]. It showed significant improvements in pain scores, edema, range of motion (ROM), and functionality among both subjects in the TENS and Sham-TENS groups with greater improvements favoring the TENS group. This study presents evidence supporting TENS providing modest pain relief and functional improvement (Level I, degree B), although the number of high-quality studies is limited.
Pharmacological Treatment—Topical Treatments
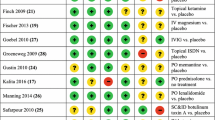
Three RCTs comprising of 64 patients compared topical treatments for CRPS, including eutectic mixture of local anesthetics (EMLA) cream [22], ketamine [23], and fatty cream with dimethyl sulfoxide (DMSO) to placebo [24] (Table 2). After 2 months of topical DMSO-containing cream along with PT, patients’ RSD-scores improved (median improvement 4 vs 3, P < 0.01) as well as VAS pain scores (2.9 vs 1, P > 0.1) [22]. Finch et al. reported improved allodynia in both treated limbs as well as in the ipsilateral forehead and hyperalgesia (30 min after application). Interestingly, Strauss et al. found that EMLA cream improved tactile resolution (t(11) = 3.98, P < 0.01) and motor function (t(10) = 2.57, P < 0.05) in the hand but not pain [24]. Overall, the efficacy of topical medications on pain intensity (Level I, degree C) and functional improvement (level I, degree C) in subjects with CRPS remain unclear with some studies showing inconsistent results of improvement versus no change when compared to placebo.
Pharmacologic Treatment—Oral, Intravenous, and Intramuscular Formulations
This section describes the treatment of CRPS with oral, intravenous (IV), and intramuscular (IM) pharmacologic formations including gabapentinoids, opioids, bisphosphonates, systemic steroids, and ketamine (Table 3). The benefits of gabapentinoids have been studied on patients (n = 586) with CRPS in two RCTs [25, 26], two prospective studies [27, 28], and one retrospective study [29]. In a RCT comparing gabapentin and placebo, van De Vusse et al. observed that gabapentin did not alleviate pain. Further, patients who were treated with gabapentin reported side effects of dizziness, somnolence, and lethargy [25]. In another RCT, Brown et al. compared gabapentin and amitriptyline. Both medications provided significant pain relief without any differences in side effects [26]. In the two prospective studies, both gabapentin and pregabalin provided pain relief but no significant improvement in function [27, 28]. Lastly, Lee et al. reported that combining IV mannitol, steroids, and oral gabapentin improved pain intensity, finger ROM, swelling, and grip strength in CRPS. Overall, participants with CRPS who received gabapentinoids achieved inconsistent pain relief (level I, degree C) and functionality (level I, degree C), with some studies reporting no benefit compared to placebo (or control arms) and some studies reporting modest benefit.
Two RCTs assessed the administration of systemic opioids in 63 patients with CRPS. One study reported that morphine (30 mg) with memantine (40 mg) was associated with greater improvement in pain and disability (habitual pain: 5.47 to 1.40; P < 0.001; movement pain: 8.03 to 2.84; P < 0.001) compared to morphine alone [30]. Harke et al. observed that after spinal cord stimulation (SCS) cessation, 8-day therapy with carbamazepine but not morphine delayed the recurrence of pain compared to placebo (P = 0.038) [31]. Overall, these studies suggest it is unclear if opioid medications offer benefit to patients with CRPS compared to placebo (level I, degree D).
Seven RCTs examined bisphosphonates for pain control in 249 patients with CRPS. Young et al. compared IV pamidronate to oral prednisolone and found significant reduction in VAS scores at 4 weeks in both groups compared to baseline, but only prednisolone improved swelling [32].
Multiple RCTs compared various IV bisphosphonates to placebo with different doses and durations [33,34,35, 36•]. Adam et al. showed that IV alendronate led to significant improvements in spontaneous pain, tenderness, swelling, and motion compared to placebo [33]. Varenna et al. demonstrated that IV clondronate significantly reduced pain and clinical global assessment compared to placebo [34]. Robinson et al. found that a single infusion of pamidronate significantly improved pain and physical function [35]. In another study [36•], four doses of IV neridronate led to significant reduction in pain intensity compared to the placebo group. The patients in the neridronate-treated group were able to discontinue NSAIDs and acetaminophen within 2 weeks compared to only 45% in the placebo group.
Manicourt et al. examined the effect of oral bisphosphonate in patients with CRPS. They showed that 8 weeks of oral alendronate improved pain, pressure tolerance, and joint mobility [37]. During an extension of this study where all patients could use oral alendronate, the patients who were previously in the placebo group showed significant improvement in the same outcomes that were achieved by the patients that were in the alendronate cohort (P < 0.05).
Varenna et al. was the only RCT examining IM bisphosphonate therapy for CRPS. They showed that the IM neridronate group achieved significantly higher pain reduction compared to placebo [38]. In an extension phase of the study, where patients initially receiving placebo treatment were treated with IV neridronate, both groups had high rates of patients with greater than 50% pain relief and significant improvement in function at 12 months [39]. Overall, these studies suggest that both oral and IV bisphosphates are effective in reducing pain intensity (level I, degree B) and improving functionality (level I, degree B) in patients with CRPS.
There were three RCTs conducted by Kalita et al. that evaluated the effects of prednisolone in CRPS [40,41,42]. These studies comprised of 154 patients. In the first study, prednisolone was superior to piroxicam with 83.3% vs 16.7% of the subjects achieving significant improvement in pain [40]. The second study divided the patients that previously responded to high-dose steroid into two groups: continue steroid or discontinue steroid. The group that discontinued steroid had a higher occurrence of symptoms compared to those who continued steroid [41]. The third study showed that CRPS patients receiving a higher dose of steroid (prednisolone 20 mg vs 40 mg) experienced significantly higher pain reduction [42]. Overall, these studies demonstrated that a short-course of systemic steroid reduced CRPS pain (level I, degree B). Functionality did not improve with systemic steroid treatment (level I, degree D).
Two RCTs comprising 79 patients evaluated the effectiveness of IV ketamine on pain from CRPS [43, 44]. Both RCTs showed that IV ketamine infusion for 4 or 10 days resulted in significant improvement in pain at the 3-month follow-up. A sub-analysis of one of the RCTs showed that there was an inverse relationship between pain intensity and motor movement parameters in the affected limb such as velocity, amplitude, and frequency [45]. These studies reported that patients receiving ketamine infusion developed higher percentages of nausea, vomiting, and psychomimetic effects compared to placebo. Overall, IV ketamine has been shown to reduced pain intensity but may manifest with intolerable side effects (level I, degree C). However, it does not appear to affect functionality (level I, degree D).
Interventional Treatments
Interventional treatments for CRPS include sympathetic plexus blocks, spinal cord stimulation (SCS), dorsal root ganglion stimulation (DRG-S), peripheral nerve stimulation (PNS), and intrathecal drug delivery system (IDDS; Table 4).
Eight RCTs assessed the efficacy of sympathetic plexus blocks for CRPS [46,47,48,49,50,51,52]. Four of the eight RCTs focused on stellate ganglion block (SGB) for upper extremity CRPS [46,47,48, 53]. Naskar et al. showed that there was no significant difference between SGB injectate with ropivacaine plus clonidine and methylprednisolone [47]. When comparing SGB to T2 paravertebral block (PVB), PVB resulted in higher success rates of reducing pain intensity, duration of pain relief, and patients’ satisfaction [48]. Toshniwal et al. demonstrated that continuous SGB and continuous infraclavicular brachial plexus block were equivocal in reducing pain and edema and improving range of motion [53].
One study investigated the benefits of thoracic sympathetic block (TSB) [49]. Rocha et al. found that TSB resulted in significant improvement in pain relief as assessed by the McGill Pain Questionnaire, Neuropathic Pain Symptom Inventory, and depression scores compared to sham procedure.
Lastly, three RCTs evaluated the effectiveness of lumbar sympathetic ganglion block [50, 51]. Meier et al. compared IV lidocaine plus lumbar sympathetic ganglion injection with normal saline versus IV normal saline plus lumbar sympathetic ganglion injection with lidocaine [50]. The authors showed that lumbar sympathetic ganglion injection with lidocaine was more effective in reducing allodynia and pain scores. Yoo et al. showed that botulotoxin type A injection into the lumbar sympathetic ganglion decreased more pain than local anesthetic injection [51]. Freitas et al. demonstrated that pulsed radiofrequency ablation of the sympathetic lumbar plexus was equally effective in reducing pain as a lumbar sympathetic ganglion block [51]. Overall, the current evidence suggests that sympathetic ganglion block is associated with meaningful pain relief in CRPS (level I, degree B).
Only one RCT was found evaluating sympathectomy for CRPS [54]. The study randomized 20 patients into two arms: radiofrequency or phenol lumbar sympathectomy. There was no statistically significant difference between groups but both had significant pain relief up to 4 months after the procedure [54]. Overall, this study showed that either radiofrequency or phenol lumbar sympathectomy may reduce pain in CRPS, although future studies supporting this association are warranted (level I, degree C).
Neuromodulation with SCS has been demonstrated to be effective in treating CPRS [76••, 77]. Six RCTs comprising of 342 patients were identified [55,56,57,58,59,60,61,62]. Kemler et al. showed that SCS improved pain and health-related quality of life compared to PT only over a 2-year period [56, 57]. However, these benefits diminished over a 5-year period [58] due to neural habituation [78] or device-related issues [79]. Interestingly, the effects on CRPS pain did not differ between different SCS settings [55, 61]. Additionally, SCS restored the quantitative sensory testing thresholds for the affected limb to that of the non-affected limb [59, 60]. Overall, dorsal-column SCS is effective in treating pain from CRPS (level I, degree B) and is also approved by the FDA for this indication.
DRG-S also carries FDA labeling for treatment of pain from CRPS and may provide substantial pain relief [80]. One RCT and one prospective study comprising of 164 patients were identified [62,63,64,65,66]. Deer et al. demonstrated that DRG-S provided a greater percentage of participants achieving ≥ 50% pain relief (PPR), quality of life, and psychological measures compared to dorsal-column SCS at the 3-month follow-up [63]. DRG-S was associated with higher cost than dorsal-column SCS [64, 65], and more subjects preferred DRG-S over dorsal-column SCS [66]. Overall, these studies highlighted that DRG-S substantially improves pain from CRPS (level I, degree B).
PNS may alleviate pain by disrupting the nociceptive afferent fibers, downregulating inflammatory mediators, and changing the local microenvironment [81,82,83]. One prospective study and three retrospective studies comprising 207 patients were identified [67,68,69,70]. These studies showed that PNS reduced pain and opioid consumption. Functional improvement varied between studies. Overall, these studies showed that PNS reduces pain intensity (level II-2, degree B) with variation in improvement in functionality (level II-2, degree C).
Two RCTs by Munts et al. compared intrathecal medications to placebo [71, 72]. Intrathecal glycine failed to show superiority over saline for pain, dystonia or global impression scores, and a single dose of intrathecal methylprednisolone did not provide pain relief in CRPS at 4 weeks of follow-up [71, 72]. Intrathecal baclofen (ITB) was administered in either a slow or rapid infusion (4-times faster) with no difference on pain [73]. Finally, when comparing intrathecal clonidine to adenosine, both treatments showed improvements in hyperalgesia and allodynia with clonidine having a threefold higher effect for pain relief [74]. Overall, the current evidence showed that only intrathecal clonidine or adenosine may improve pain intensity although future studies are warranted to support this association (level I, degree C). There was no report of functional improvement (level I, degree I).
Discussion
In this systematic review, we described modest to moderate improvement in pain intensity from PT, OT, MT, acupuncture, and TENS therapy, although changes in functionality were inconsistent. Topical medications such as EMLA and Ketamine cream were associated with decreased allodynia and hyperalgesia. There were mixed results supporting gabapentinoids for reduction of pain in CRPS. It was unclear whether systemic opioid alone was able to provide pain relief in CRPS because the study design of included studies reported adjuvant therapy such as memantine or carbamazepine added to systemic morphine. There was no study comparing morphine to placebo. There were positive outcomes associated with IM and IV bisphosphonates. Patients showed modest to moderate improvement in pain intensity and functionality. Systemic steroid provided moderate short-term pain reduction, although pain recurred after 1 month of discontinuation. Similarly, ketamine was able to provide short-term pain relief, although adverse effects were commonly reported. In terms of interventional therapy, no benefit was reported for intrathecal drug delivery system. Sympathetic ganglion block, sympathectomy, SCS, DRG-S, and PNS were associated with modest to moderate improvement in CRPS pain intensity.
The findings in this systematic review are consistent with the recommendations from prior reviews in the literature [84]. From a therapeutic approach, we recommend starting with conservative therapies such as PT with aerobic exercises/pain exposure, OT, MT, acupuncture, and TENS. These therapies may need to be implemented concurrently alongside other treatment modalities to decrease pain and restore function (Fig. 2). In refractory cases of CRPS, clinicians may consider treating the patient with conservative therapies, medications, and interventional therapy at the same time. Neuromodulation and sympathectomy are considered last-resort therapies given its invasive nature, high cost, and potential adverse events. Opportunities for future research exist for treatment modalities with limited RCT data such as PNS therapy and neuropathic pain medications (e.g., gabapentinoids, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors).
Conclusion
In conclusion, this systematic review equips the clinician with important updates on conservative, pharmacologic, and interventional treatment modalities for CRPS-related pain. There is level I evidence supporting modest to moderate improvement in pain intensity from physical therapy, occupational therapy, massage therapy, acupuncture, and TENS. EMLA and ketamine cream were associated with decreased allodynia and hyperalgesia. Intramuscular or intravenous bisphosphonate therapy may achieve modest to moderate improvement in pain intensity and functionality. Systemic steroid and ketamine provided clinically significant pain reduction, although it was of short duration. Interventional therapy, including sympathetic ganglion block, sympathectomy, dorsal column spinal cord stimulation, dorsal root ganglion stimulation, and peripheral nerve stimulation, may provide modest to moderate improvement in pain with although the level of evidence was limited.
Data Availability
Data analyzed in this manuscript are available upon request to the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Misidou C, Papagoras C. Complex regional pain syndrome: an update. Mediterr J Rheumatol. 2019;30(1):16–25.
Eldufani J, Elahmer N, Blaise G. A medical mystery of complex regional pain syndrome. Heliyon. 2020;6(2):e03329.
Goebel A, et al. The Valencia consensus-based adaptation of the IASP complex regional pain syndrome diagnostic criteria. Pain. 2021;162(9):2346–8.
Kim Y-D. Diagnosis of complex regional pain syndrome. Ann Clin Neurophysiol. 2022;24(2):35–45.
• de Mos M, et al. Medical history and the onset of complex regional pain syndrome (CRPS). Pain. 2008;139(2):458–66. This study provides insights into the medical history prior to the onset of complex regional pain syndrome and identifies potential risk factors.
Zangrandi A, Allen Demers F, Schneider C. Complex regional pain syndrome a comprehensive review on neuroplastic changes supporting the use of non-invasive neurostimulation in clinical settings. Front Pain Res (Lausanne). 2021;2:732343.
Elsamadicy AA, et al. Prevalence and cost analysis of complex regional pain syndrome (CRPS): a role for neuromodulation. Neuromodulation. 2018;21(5):423–30.
Lee BH, et al. Physical therapy and cognitive-behavioral treatment for complex regional pain syndromes. J Pediatr. 2002;141(1):135–40.
Topcuoglu A, et al. The effect of upper-extremity aerobic exercise on complex regional pain syndrome type I: a randomized controlled study on subacute stroke. Top Stroke Rehabil. 2015;22(4):253–61.
Barnhoorn KJ, et al. Pain exposure physical therapy (PEPT) compared to conventional treatment in complex regional pain syndrome type 1: a randomised controlled trial. BMJ Open. 2015;5(12):e008283.
Den Hollander M, et al. Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. Pain. 2016;157(10):2318–29.
Oerlemans HM, Goris RJA, Oostendorp RAB. Physical therapy and complex regional pain syndrome type I/reflex sympathetic dystrophy: a randomized controlled clinical trial. Nederlands tijdschrift fysiotherapie. 2003;113(Special):15–9.
Oerlemans HM, et al. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Arch Phys Med Rehabil. 2000;81(1):49–56.
Cacchio A, et al. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair. 2009;23(8):792–9.
Pervane Vural S, et al. Effects of mirror therapy in stroke patients with complex regional pain syndrome type 1: a randomized controlled study. Arch Phys Med Rehabil. 2016;97(4):575–81.
Moseley GL. Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? Randomised Clin Trial Pain. 2005;114(1–2):54–61.
Korpan MI, et al. Acupuncture in the treatment of posttraumatic pain syndrome. Acta Orthop Belg. 1999;65(2):197–201.
Li N, et al. Therapeutic effect of acupuncture and massage for shoulder-hand syndrome in hemiplegia patients: a clinical two-center randomized controlled trial. J Tradit Chin Med. 2012;32(3):343–9.
Liu H, Zhang C. 60 cases of shoulder-arm syndrome treated by electroacupuncture at Bingfeng (SI 12). Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan / sponsored by All-China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine. 1998;18(4):256–258.
Zheng J, et al. A clinical study on acupuncture in combination with routine rehabilitation therapy for early pain recovery of post-stroke shoulder-hand syndrome. Exp Ther Med. 2018;15(2):2049–53.
Bilgili A, et al. The effectiveness of transcutaneous electrical nerve stimulation in the management of patients with complex regional pain syndrome: a randomized, double-blinded, placebo-controlled prospective study. J Back Musculoskelet Rehabil. 2016;29(4):661–71.
Strauss S, et al. Functional imaging of somatosensory finger representation and intracortical inhibition modulation in complex regional pain syndrome. Clin Neurophysiol. 2015;126(8):e83.
Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: a double-blind placebo-controlled trial of topical ketamine. Pain. 2009;146(1–2):18–25.
Zuurmond WW, et al. Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream. Acta Anaesthesiol Scand. 1996;40(3):364–7.
van de Vusse AC, et al. Randomised controlled trial of gabapentin in complex regional pain syndrome type 1 [ISRCTN84121379]. BMC Neurol. 2004;4(no pagination).
Brown S, et al. A randomized controlled trial of amitriptyline versus gabapentin for complex regional pain syndrome type I and neuropathic pain in children. Scand J Pain. 2016;13:156–63.
Tan AK, et al. The effect of gabapentin in earlier stage of reflex sympathetic dystrophy. Clin Rheumatol. 2007;26(4):561–5.
de la Calle JL, et al. Add-on treatment with pregabalin for patients with uncontrolled neuropathic pain who have been referred to pain clinics. Clin Drug Investig. 2014;34(12):833–44.
Lee SK, et al. Four treatment strategies for complex regional pain syndrome type 1. Orthopedics. 2012;35(6):e834–42.
Gustin SM, et al. NMDA-receptor antagonist and morphine decrease CRPS-pain and cerebral pain representation. Pain. 2010;151(1):69–76.
Harke H, et al. The response of neuropathic pain and pain in complex regional pain syndrome I to carbamazepine and sustained-release morphine in patients pretreated with spinal cord stimulation: a double-blinded randomized study. Anesth Analg. 2001;92(2):488–95.
Eun Young H, Hyeyun K, Sang HI. Pamidronate effect compared with a steroid on complex regional pain syndrome type I: pilot randomised trial. Neth J Med. 2016;74(1):30–5.
Adami S, et al. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis. 1997;56(3):201–4.
Varenna M, et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol. 2000;27(6):1477–83.
Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med. 2004;5(3):276–80.
• Varenna M, et al. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology (Oxford). 2013;52(3):534–42. This randomized controlled trial shows that intramuscular bisphosphonate therapy may be associated with clinically relevant benefit in acute complex regional pain syndrome compared to placebo administration.
Manicourt DH, et al. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum. 2004;50(11):3690–7.
Varenna M, et al. Intramuscular neridronate for the treatment of complex regional pain syndrome type 1: a randomized, double-blind, placebo-controlled study. Ther Adv Musculoskelet Dis. 2021;13:1759720X211014020.
Varenna M, et al. Long-term efficacy and safety of neridronate treatment in patients with complex regional pain syndrome type 1: a pre-specified, open-label, extension study. Ther Adv Musculoskelet Dis. 2022;14(no pagination).
Kalita J, Vajpayee A, Misra UK. Comparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: a randomized controlled trial. QJM. 2006;99(2):89–95.
Kalita J, et al. Long-term prednisolone in post-stroke complex regional pain syndrome. Pain Physician. 2016;19(8):565–74.
Kalita J, et al. Prednisolone 20 mg vs 40 mg in complex regional pain syndrome type I: A randomized controlled trial. J Clin Neurosci. 2023;113:108–13.
Schwartzman RJ, et al. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147(1–3):107–15.
Sigtermans MJ, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145(3):304–11.
Schilder JCM, et al. Pain relief is associated with improvement in motor function in complex regional pain syndrome type 1: secondary analysis of a placebo-controlled study on the effects of ketamine. J Pain. 2013;14(11):1514–21.
Yoo SD, et al. Efficacy of ultrasonography guided stellate ganglion blockade in the stroke patients with complex regional pain syndrome. Ann Rehabil Med. 2012;36(5):633–9.
Naskar S, et al. A comparison of analgesic efficacy and safety of clonidine and methylprednisolone as additives to 0.25% ropivacaine in stellate ganglion block for the treatment of complex regional pain syndrome: a prospective randomised single blind study. Korean J Pain. 2023;36(2):216–29.
Kim YH, et al. A prospective, randomized cross-over trial of T2 paravertebral block as a sympathetic block in complex regional pain syndrome. Pain Physician. 2019;22(5):E417–24.
Rocha RdO, et al. Thoracic sympathetic block for the treatment of complex regional pain syndrome type I: a double-blind randomized controlled study. Pain. 2014;155(11):2274–81.
Meier PM, et al. Lumbar sympathetic blockade in children with complex regional pain syndromes: a double blind placebo-controlled crossover trial. Anesthesiology. 2009;111(2):372–80.
Yoo Y, et al. Botulinum toxin type A for lumbar sympathetic ganglion block in complex regional pain syndrome: a randomized trial. Anesthesiology. 2022;136(2):314–25.
Freitas TS, Deusdara R, Kessler I. Pulsed radiofrequency of sympathetic lumbar plexus versus sympathetic block in the management of lower limb complex regional pain syndrome type. Stereotact Funct Neurosurg. 2013;91(107).
Toshniwal G, et al. Management of complex regional pain syndrome type I in upper extremity-evaluation of continuous stellate ganglion block and continuous infraclavicular brachial plexus block: a pilot study. Pain Med. 2012;13(1):96–106.
Manjunath PS, et al. Management of lower limb complex regional pain syndrome type 1: an evaluation of percutaneous radiofrequency thermal lumbar sympathectomy versus phenol lumbar sympathetic neurolysis–a pilot study. Anesth Analg. 2008;106(2):647–9 (table of contents).
Canos-Verdecho A, et al. Randomized prospective study in patients with complex regional pain syndrome of the upper limb with high-frequency spinal cord stimulation (10-kHz) and low-frequency spinal cord stimulation. Neuromodulation. 2021;24(3):448–58.
Kemler MA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618–24.
Kemler MA, et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55(1):13–8.
Kemler MA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108(2):292–8.
Kemler MA, et al. Impact of spinal cord stimulation on sensory characteristics in complex regional pain syndrome type I: a randomized trial. Anesthesiology. 2001;95(1):72–80.
Kriek N, et al. Allodynia, hyperalgesia, (quantitative) sensory testing and conditioned pain modulation in patients with complex regional pain syndrome before and after spinal cord stimulation therapy. Neuromodulation. 2023;26(1):78–86.
Kriek N, et al. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain. 2017;21(3):507–19.
Levy RM, et al. Therapy habituation at 12 months: spinal cord stimulation versus dorsal root ganglion stimulation for complex regional pain syndrome type I and II. J Pain. 2020;21(3–4):399–408.
Deer TR, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–81.
Mekhail N, et al. Paresthesia-free dorsal root ganglion stimulation: an ACCURATE study sub-analysis. Neuromodulation. 2020;23(2):185–95.
Mekhail N, et al. Cost-effectiveness of dorsal root ganglion stimulation or spinal cord stimulation for complex regional pain syndrome. Neuromodulation. 2021;24(4):708–18.
van Bussel CM, Stronks DL, Huygen FJPM. Dorsal column stimulation vs. dorsal root ganglion stimulation for complex regional pain syndrome confined to the knee: patients’ preference following the trial period. Pain Practice. 2018;18(1):87–93.
Bouche B, et al. Peripheral nerve stimulation of brachial plexus nerve roots and supra-scapular nerve for chronic refractory neuropathic pain of the upper limb. Neuromodulation. 2017;20(7):684–9.
Buwembo J, et al. Direct sciatic nerve electrical stimulation for complex regional pain syndrome type 1. Neuromodulation. 2021;24(6):1075–82.
Chmiela MA, et al. Direct peripheral nerve stimulation for the treatment of complex regional pain syndrome: a 30-year review. Neuromodulation. 2021;24(6):971–82.
Frederico TN, da Silva Freitas T. Peripheral nerve stimulation of the brachial plexus for chronic refractory CRPS pain of the upper limb: description of a new technique and case series. Pain Medicine. 2020;21(Suppl 1):S18–26.
Munts AG, et al. Intrathecal glycine for pain and dystonia in complex regional pain syndrome. Pain. 2009;146(1–2):199–204.
Munts AG, et al. Efficacy and safety of a single intrathecal methylprednisolone bolus in chronic complex regional pain syndrome. Eur J Pain. 2010;14(5):523–8.
van der Plas AA, et al. The lack of efficacy of different infusion rates of intrathecal baclofen in complex regional pain syndrome: a randomized, double-blind, crossover study. Pain Med. 2011;12(3):459–65.
Rauck RL, North J, Eisenach JC. Intrathecal clonidine and adenosine: effects on pain and sensory processing in patients with chronic regional pain syndrome. Pain. 2015;156(1):88–95.
Scholz-Odermatt SM, et al. Direct health care cost and work incapacity related to complex regional pain syndrome in Switzerland: a retrospective analysis from 2008 to 2015. Pain Med. 2019;20(8):1559–69.
•• D’Souza RS, et al. The state-of-the-art pharmacotherapeutic options for the treatment of chronic non-cancer pain. Expert Opin Pharmacother. 2022;23(7):775–89. This comprehensive review describes multimodal pharmacotherapeutic analgesia strategies for chronic pain treatment including anti-inflammatory agents, opioid medications, anti-convulsants, anti-depressants, sodium channel blockers, cannabinoids, and alpha-2-receptor blockers.
ElSaban M, et al. Physical functioning following spinal cord stimulation: a systematic review and meta-analysis. Reg Anesth Pain Med. 2023;48(6):302–11.
D’Souza RS, Her YF. Stimulation holiday rescues analgesia after habituation and loss of efficacy from 10-kilohertz dorsal column spinal cord stimulation. Reg Anesth Pain Med. 2022;47(12):722–7.
D’Souza RS, et al. Adverse events associated with 10-kHz dorsal column spinal cord stimulation: a 5-year analysis of the Manufacturer and User Facility Device Experience (MAUDE) database. Clin J Pain. 2022;38(5):320–7.
D’Souza RS, et al. Dorsal root ganglion stimulation for lower extremity neuropathic pain syndromes: an evidence-based literature review. Adv Ther. 2022;39(10):4440–73.
Abd-Elsayed A, D’Souza RS. Peripheral nerve stimulation: the evolution in pain medicine. Biomedicines. 2021;10(1).
Strand NH, et al. Mechanism of action of peripheral nerve stimulation. Curr Pain Headache Rep. 2021;25(7):47.
Char S, et al. Implantable peripheral nerve stimulation for peripheral neuropathic pain: a systematic review of prospective studies. Biomedicines. 2022;10(10).
Harden RN, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 5th edition. Pain Med. 2022;23(Suppl 1):S1-S53.
Acknowledgements
We would like to thank Leslie C. Hassett M.L.S, A.H.I.P. for developing the search strategy.
Author information
Authors and Affiliations
Contributions
Yeng F. Her, Eva Kubrova, Marissa Dombovy-Johnson, Mariam ElSaban, Karson Mostert, and Ryan D’Souza contributed to the data acquisition, data analysis, table and figure generation, manuscript draft composition, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
RSD receives investigator-initiated grant funding paid to his institution from Nevro Corp and Saol Therapeutics. The other co-authors declare no conflicts of interest.
Human and Animal Rights Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Her, Y.F., Kubrova, E., Dombovy-Johnson, M. et al. Complex Regional Pain Syndrome: Updates and Current Evidence. Curr Phys Med Rehabil Rep 12, 50–70 (2024). https://doi.org/10.1007/s40141-023-00426-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-023-00426-2