Abstract
Dorsal root ganglion stimulation (DRG-S) is a form of selective neuromodulation therapy that targets the dorsal root ganglion. DRG-S offers analgesia in a variety of chronic pain conditions and is approved for treatment of complex regional pain syndrome (CRPS) by the US Food and Drug Administration (FDA). There has been increasing utilization of DRG-S to treat various neuropathic pain syndromes of the lower extremity, although evidence remains limited to one randomized controlled trial and 39 observational studies. In this review, we appraised the current evidence for DRG-S in the treatment of lower extremity neuropathic pain using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) criteria. The primary outcome was change in pain intensity after DRG-S compared to baseline. We stratified presentation of results based of type of neuropathy (CRPS, painful diabetic neuropathy, mononeuropathy, polyneuropathy) as well as location of neuropathy (hip, knee, foot). Future powered randomized controlled trials with homogeneous participants are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dorsal root ganglion stimulation (DRG-S) is a form of selective neuromodulation therapy that targets the dorsal root ganglion and offers analgesia in a variety of chronic pain conditions. |
DRG-S is currently approved by the US Food and Drug Administration for the treatment of neuropathic pain associated with complex regional pain syndrome (CRPS) and/or causalgia in the groin and lower extremity. |
The highest level of evidence is demonstrated with DRG-S use for CRPS of the lower extremity, although the quality of evidence was downgraded to “low” because of risk of bias, imprecision, heterogeneity, and indirectness from included observational studies. |
There is very low-quality evidence demonstrating promising results for DRG-S in painful diabetic neuropathy, focal neuropathy, and polyneuropathy. |
Efficacy of DRG-S for refractory postsurgical neuropathic pain of the groin (e.g., post-herniorrhaphy) or of the knee (e.g., post-total knee arthroplasty) has been demonstrated in small-scale observational studies. |
Introduction
The dorsal root ganglion (DRG) contains a collection of cell bodies of primary sensory, pseudo-unipolar neurons in the lateral epidural space of the spinal foramen [1]. DRG neurons are involved in the transduction of pain to the central nervous system (CNS), serving as a filter for propagation of afferent signals to the dorsal horn [1]. DRG stimulation (DRG-S) is a form of selective neuromodulation therapy that targets these primary sensory neurons and offers analgesia in a variety of chronic pain conditions [2].
DRG-S was first described in a case report in 1998, when a conventional spinal cord stimulator lead was used to target the DRG in a patient with discogenic low back pain and resulted in 69% pain relief [3]. In 2009, a DRG-specific stimulator lead was developed with a much smaller diameter, increased flexibility, and reduced contact size. This design allowed for precise targeting of nerve fibers that innervate the targeted painful regions without nonspecifically recruiting uninvolved dermatomes, even with areas that are typically more difficult to target with dorsal column spinal cord stimulation (SCS) like the groin and the foot. Because the DRG only has a thin layer of cerebrospinal fluid around it, DRG-S is achieved with a lower electrical current, is less affected by positional changes, possesses more stability, and offers superior electrical efficiency and energy consumption compared to dorsal column SCS [4]. However, DRG-S may have notable disadvantages including limited applicability in more widespread pain, difficulty of percutaneous placement, and difficulty or unfeasibility of placement in cases with neuroforaminal stenosis.
In 2011, the European Union and Australia approved the use of DRG-S after a multicenter study showed favorable efficacy in 76.5% of subjects with chronic, intractable neuropathic pain in trunk, sacrum, or lower limbs [5]. Subsequently, a multicenter, comparative, prospective, controlled efficacy trial showed a statistically significant higher rate of treatment success, improvement in quality of life, functional status, and psychological disposition in patients with complex regional pain syndrome (CRPS) and causalgia when treated with DRG-S in comparison to SCS treatment [6]. With these data, the US Food and Drug Administration (FDA) approved DRG-S in February of 2016 for the treatment of pain associated with CRPS of the lower extremities [7].
Although DRG-S has been practiced since 1998 [3], most of the evidence supporting DRG-S for neuropathic pain is recent. Furthermore, DRG-S is only approved for CRPS and/or peripheral causalgia in the groin and lower limb. Yet its utilization for other unapproved painful conditions of the lower extremity, such as painful diabetic neuropathy [8], persistent postsurgical pain [9], and mononeuropathies [10], is expanding and warrants evidence appraisal. Thus, the aim of this review is to assess the change in pain intensity in lower extremity neuropathic pain conditions after DRG-S, and to appraise the level of evidence for this outcome. We stratify presentation of results based of type of neuropathy, as well as location of neuropathy. Finally, we also highlight the mechanism of action of DRG-S in the treatment of neuropathic pain. This review is timely and relevant as it provides clinicians an updated appraisal of evidence for several clinical indications. In light of habituation [11] and loss of efficacy [12,13,14] from long-term dorsal column spinal cord stimulation, this review highlights yet another unique modality of neuromodulation that can be offered to patients to salvage analgesia, physical functionality, and patient satisfaction [15]. Finally, as researchers continue to elucidate the mechanisms of action from neuromodulation that lead to analgesia, DRG-S will be a primary focus due to the role of DRG neurons in pain transduction, afferent signal filtering, and windup phenomena [1].
Methods
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy
A comprehensive search of several databases from each database’s inception to March 23, 2022, consisting of any language, was conducted. The databases included Ovid MEDLINE®, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian (LJP) with input from the study’s principal investigator (RSD). Controlled vocabulary supplemented with keywords was used to search for DRG-S for treatment of lower extremity neuropathic pain. The search strategy listing all search terms used and how they are combined is available in the Supplementary Material.
Study Selection and Data Extraction
Included studies abided to the following criteria: any study design (case report, case series, retrospective or prospective observational studies, or randomized controlled trials) that involved patients receiving DRG-S for lower extremity neuropathic pain and included patient-reported measurements of pain intensity or pain relief after DRG-S. Exclusion criteria consisted of the following: non-peer-reviewed studies, review or meta-analysis articles, nonhuman studies, and unpublished clinical trials. The primary outcome was change in pain intensity after DRG-S compared to baseline. Specific outcome measures may consist of calculated or patient-reported percentage pain relief, as well as visual analog pain score or numeric rating scale score before and after DRG-S. Secondary outcomes included changes in physical functioning, emotional functioning, patient satisfaction, and adverse events.
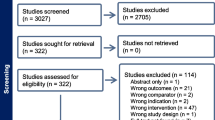
Two authors (RSD and EK) independently selected abstracts along with full-text articles from the aforementioned databases, while a third author (AA) resolved any discrepancies (Fig. 1). The following data was extracted: (1) study characteristics (indication, DRG-S lead location, study design, funding), (2) participant demographics (sample size, average age, type of neurological impairment), (3) outcome of interest (change in pain intensity after DRG-S compared to baseline).
Assessment of Risk of Bias
The Newcastle–Ottawa Scale (NOS) was used to assess risk of bias in observational studies (containing over 10 participants) [5]. The Cochrane risk of bias assessment tool was used to assess risk of bias for randomized controlled trials (RCTs) [5, 16]. Risk of bias was only performed for studies assessing DRG-S for a specific neuropathic pain diagnosis (not solely location of pain). The NOS comprises the following three domains: selection, comparability, and exposure/outcome. Each specific NOS item can be assigned a maximum of 1 star, and a maximum of 2 stars can be given for the comparability domain. Studies with more stars for each domain have lower risk for bias for those respective domains. The Cochrane risk of bias tool assessed bias risk in the following domains: selection, performance, detection, attrition, reporting, and other biases. Each domain was assigned a grade of low risk, high risk, or unclear risk. For each included study, two authors (RSD and YH) independently assessed for risk of bias with a third author (AA) arbitrating any disputes.
Quality of Evidence
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) quality assessment criteria [17] were used to assess the level of evidence for DRG-S use for each specific neuropathic pain diagnosis. GRADE certainty of evidence can be rated as one of the following four options: high, moderate, low, or very low. Although RCTs receive a high-level score, the level of evidence can be downgraded on the basis of deficiencies in domains of risk of bias [18], inconsistency [19], indirectness [20], imprecision [21], and publication bias [22].
Results
Complex Regional Pain Syndrome
Per the Budapest criteria, CRPS is a diagnosis characterized by pain that is disproportionate to the inciting event associated with abnormal sensory (allodynia), as well as vasomotor, sudomotor, and/or motor/trophic symptoms [23]. One RCT [6] and 11 observational studies [5, 10, 16, 24,25,26,27,28,29,30,31] described DRG-S for treatment of pain from CRPS (Table 1). The ACCURATE RCT [6] demonstrated that DRG-S was non-inferior (p < 0.0001) and superior (p < 0.0004) to traditional dorsal column SCS through 12 months of follow-up for lower extremity CRPS pain intensity. When the primary endpoint was assessed at 3 months (50% reduction in visual analog scale [VAS] from pre-implant levels), 56 of 69 (81.2%) participants receiving DRG-S achieved treatment success versus 39 of 70 (55.7%) participants receiving dorsal column SCS (statistical non-inferiority p < 0.0001, statistical superiority p < 0.0004). Similarly, 49 of 66 (74.2%) participants in the DRG-S cohort achieved the primary endpoint at 12 months versus 35 of 66 (53.0%) participants in the SCS cohort (statistical non-inferiority p < 0.0001, statistical superiority p < 0.0004). The DRG-S cohort also reported higher quality of life, functional status, and emotional functioning metrics compared to those receiving dorsal column SCS. Importantly, participants receiving DRG-S reported less postural variation of paresthesias and less non-specific, extraneous stimulation in non-painful areas compared to those receiving dorsal column SCS.
Findings from the pivotal ACCURATE trial are further supported by data from five prospective observational studies [5, 16, 24,25,26], four cases series [10, 27,28,29], and two case reports [30, 31]. In total, these observational studies comprised 148 participants with CRPS type I and 188 participants with CRPS type II. Huygen et al. [25] reported a multicenter, prospective study that enrolled participants with failed back surgery syndrome, peripheral neuropathy, and CRPS. Eleven CRPS type I participants (46.8%) and 13 CRPS type II (43.7%) participants reported pain relief at 12 months. Gravius et al. [24] conducted a prospective study on participants with CRPS type I, who achieved 61.3% pain reduction along with improved mood and sleep metrics at 3 months post-implant. Morgalla et al. [36] performed a prospective study that enrolled 62 participants with CRPS type II with 51 receiving permanent DRG-S. The study reported improvement in mean VAS scores (8.0–4.5), function (Brief Pain Inventory, 76–30; Pain Disability Index, 45–23), and mood (Beck Depression Inventory, 36–21) at 36-month follow-up. Liem et al. [5] reported that eight participants with CRPS type I with permanent DRG-S had reduced pain by 56% at 12 months. Knife et al. [16] studied the effects of DRG-S on quantitative sensory threshold (QST) testing in participants with CRPS type I, and reported improved warmth, tactile, and vibration QSTs along with normalization of pain sensory thresholds after DRG-S implant. These positive outcomes from prospective observational studies supporting DRG-S for CRPS are further reinforced by data from case series and reports [10, 27,28,29,30,31].
In summary, the evidence base for DRG-S for the treatment of CRPS type I or CRPS type II includes one RCT and 11 observational studies reporting outcomes between 3 and 36 months. There was low-quality GRADE evidence that DRG-S reduced pain and disability in participants with CRPS type I or CRPS type II. According to the GRADE approach, the evidence quality was initially rated as “moderate quality” evidence based on the ACCURATE trial [6]. With inclusion of observational studies, this was downgraded to “low quality” because of risk of bias, imprecision, and indirectness (Fig. 2 and Table 2). Furthermore, the participants in these studies were heterogenous. Aside from the ACCURATE trial, the sample size of participants in each study was low and underpowered to detect significant differences between the groups.
Painful Diabetic Neuropathy
Painful diabetic neuropathy (PDN) is defined as a symmetric, length-dependent sensorimotor polyneuropathy attributable to metabolic and microvascular alterations as a result of chronic hyperglycemia exposure and cardiovascular risk covariates [43]. This can be a progressive and debilitating condition that can cause painful paraesthesias, with only one-third of patients typically achieving clinically significant pain relief from conventional therapy [44]. Neuromodulation interventions are becoming more common modalities utilized in the treatment of PDN [45,46,47]. Two retrospective studies [8, 48] and one case report [49] described DRG-S for treatment of PDN (Table 3).
Eldabe et al. [8] performed a retrospective study of ten participants with chronic intractable PDN who trialed DRG-S using up to four quadripolar percutaneous leads between L2 and L5. Of the seven patients who proceed to implantation, five patients followed to six months reported a mean VAS reduction of 49.4 mm, and four patients followed to twelve months reported a mean VAS reduction of 48.2 mm. Overall, this study reported a 70% success rate for trial-to-implant ratio and clinically significant pain relief in most patients followed to 12 months. Similarly, Falowski et al. [48] performed a retrospective analysis in which eight patients with chronic intractable peripheral neuropathy of the lower extremities underwent DRG-S. Two of these patients had PDN, with one achieving over 70% pain relief after bilateral L5 DRG lead placement after 6 weeks, and the other patient experiencing 100% pain relief after lead placement unilaterally at the left L4 and L5 levels. Finally, Chapman et al. [49] published a case report of a 61-year-old man with PDN and low back pain. He underwent a 7-day trial of unilateral DRG stimulation at the right T12 and S1, which allowed the untreated side to serve as a comparison. This resulted in significant pain relief in both feet and low back pain, with a VAS reduction from 8 to 1 cm for his back pain, and from 9 cm to 0 for his feet pain. This patient also had improvements in quality of life, physical function, emotional function, ambulation, skin color, and numbness in his feet.
In summary, the available evidence for DRG-S for PDN includes two retrospective case series and a case report, with study outcomes between 7 days and 12 months. Overall, there was very low-quality GRADE evidence that DRG-S reduced pain intensity for participants with PDN. Criteria that lowered the evidence level included study methodology limitations, indirectness due to varying patient diagnoses, and imprecision from small sample sizes.
Mononeuropathy and Focal Neuropathy
One retrospective study [50] and two case series [48, 51] reported outcomes from DRG-S in participants with focal neuropathy or mononeuropathy (Table 4). Kretzschmar et al. [50] reported that 23 of 27 participants with traumatic upper extremity and lower extremity injury underwent DRG-S implant after a successful DRG-S trial providing greater than 50% pain relief. At 36 months follow-up, pain intensity was reduced by a mean of 73%. Functional scores for Short Form-12 mental component summary and Short Form-12 physical component summary were improved from 34.1 to 43.6 and 42.3 to 50.0, respectively. Twenty of 21 participants were opioid-free at 36 months.
Zuidema et al. [51] reported two cases of focal neuropathy of the lateral femoral cutaneous nerve (LFCN), and ilioinguinal nerve and genitofemoral nerve (IGN and GFN). After DRG-S implant, the participant with LFCN neuropathy reported 90% pain relief after 2 months. The participant with neuropathy at the IGN and GFN reported 100% pain relief at 3 months after DRG-S. Finally, Falowski et al. [48] reported one case of chronic intractable left L5 radiculopathy that was treated with DRG-S. The participant reported 100% pain relief at 6 weeks and had stopped taking baseline non-steroidal anti-inflammatory drugs.
Other Idiopathic Peripheral Neuropathies and Polyneuropathies
A prospective pilot study by Koetsier et al. [55] evaluated DRG-S on nine patients with intractable large fiber polyneuropathy (Table 5). Etiologies included type II diabetes, chemotherapy-induced peripheral neuropathy (CIPN), chronic inflammatory demyelinating polyradiculoneuropathy, and idiopathic. In seven of the nine patients who underwent DRG-S implantation, daytime pain at 6 months decreased from 7.0 to 3.0, nighttime pain decreased from 5.4 to 1.0, and peak pain decreased from 9.0 to 4.0. The etiologies of polyneuropathy in this study were heterogeneous and there was no subgroup analysis between etiologies and outcome measures. However, the authors reported that the two patients with PDN had similar pain relief in comparison to other etiologies.
Grabnar et al. [56] reported DRG-S in a 50-year-old woman with a history of breast cancer and lower extremity CIPN. She had a successful 7-day DRG-S trial, achieving a decrease in VAS from 8 to 0, and her Douleur Neuropathique 4 (DN4) questionnaire score improved from 8/10 to 2/10. She has persistent pain relief 3 years later.
Overall, the body of literature for use of DRG-S in polyneuropathy is limited to case reports and prospective observational study data. There was very low-quality GRADE evidence supporting DRG-S use in reducing pain and impairment in polyneuropathy. According to the GRADE approach, the evidence quality for Koetsier et al. was initially considered as low, but ultimately was downgraded on the basis of indirectness with differing diagnoses of participants, notable methodological biases, imprecision from small sample sizes, and inclusion of case reports.
Groin Pain
A systematic review concluded that there is moderate evidence for DRG-S in treatment of neuropathic pain of the trunk or lower extremities, although it does not specifically address pain confined to the groin [64]. Advantages of DRG-S over dorsal column SCS include targeting discrete areas, maintenance of efficacy with postural changes, and lack of paresthesia in non-targeted areas [6]. These unique features of DRGS posit it to be especially useful in post-herniorrhaphy groin pain, a notoriously challenging region to target via dorsal column SCS [65,66,67]. Inguinal hernia repair is a common procedure, and the literature suggests that between 5% and 25% of patients will manifest post-herniorrhaphy neuralgia [65, 68]. Thus, the frequency of hernia repair procedures along with the percentage of patients afflicted by post-herniorrhaphy neuralgia suggests a need for escalation of treatment options in patients not amenable to medical management only. In addition to post-herniorrhaphy groin pain, DRG-S may be a viable and effective treatment option for other painful etiologies affecting the groin such as CRPS, structural damage to the ilioinguinal, iliohypogastric, or genitofemoral nerves, post-vasectomy neuralgia, and chronic pelvic pain syndrome [66].
Schu et al. [9] conducted a multicenter, retrospective study of patients who underwent DRG-S for chronic intractable, neuropathic groin pain. Of the 29 patients who underwent DRG-S trial, 25 achieved 50% or greater pain reduction and underwent implantation. After a median follow-up of 26 weeks, 19 of 23 patients achieved greater than 50% reduction in pain intensity, with 11 of 19 patients reporting pain reduction greater than 80%. Similarly, another multicenter, retrospective study [68] reviewed 32 patients with chronic neuropathic groin pain and reported a mean reduction of 68.6% in NRS after DRG-S trial. Lead placement was performed at T12–L2 for most patients; however, the range of levels targeted included T9–S3. After analysis of varying lead arrays, the authors recommended that for post-herniorrhaphy patients, the optimal lead arrangement was either T12, L1, or L2 in combination with either L2 or L3 [68]. These findings are concordant with Morgalla et al. [10] reporting promising long-term results at 3-year follow-up in a prospective cohort study of 34 patients with chronic groin pain treated with DRG-S.
In summary, there is limited evidence pooled from both retrospective and prospective observational studies highlighting that DRG-S may be an effective treatment modality for chronic, intractable neuropathic groin pain with most patients obtaining relief when leads were placed at the T12–L2 level.
Knee Pain
Total knee arthroplasty (TKA) is one of the most common surgeries with over 700,000 performed in the USA annually. Unfortunately, 8–34% of post-TKA patients experience a complicated postsurgical course with persistent neuropathic postsurgical pain (NPP) [66, 67]. DRG-S may offer a viable treatment modality for patients with persistent neuropathic knee pain refractory to conventional medical management.
Hunter et al. [68] conducted a multicenter, retrospective registry study of DRG-S for neuropathic knee pain. Their results of 23 patients showed 66.1% mean pain relief and 30% of patients reported greater than 80% improvement in pain intensity. Their analysis of a variety of lead combinations found that DRG-S provided a pain score reduction of 86.4% when targeting the L4 DRG and 74% reduction when targeting the L3 DRG. Thus, the study recommended targeting both the L3 and L4 DRG for treatment of neuropathic knee pain; if the implanting provider desires to only place one lead, the authors reported that preference should be given to L4 DRG placement given its slightly higher efficacy in pain reduction compared to L3 DRG placement [68].
Similarly, Martin et al. [69] conducted a secondary analysis of a prospective study of 14 patients with neuropathic knee pain. Of 14 patients, 12 had a successful trial and received a permanent implant, including five patients with postsurgical knee pain and seven patients with pain secondary to traumatic injury. At a mean follow-up of 34 months, they found an 80% median improvement in pain score and a 54% mean reduction in oral morphine equivalents. Lead placement was determined during DRG-S trial, with eight patients achieving a positive response to L3 DRG-S alone, one patient from L4 DRG-S alone, and three patients requiring combined L3 and L4 DRG-S to adequately cover their painful region.
In a prospective, crossover study comparing DRG-S and dorsal column SCS for CRPS confined to the knee, 10 of 12 (83%) patients preferred DRG-S while two of 12 (17%) patients chose to proceed with dorsal column SCS [33]. The lead placement for DRG-S was at both the L3 and L4 DRGs for all patients [33]. This crossover study concluded that in patients with chronic CRPS confined to the knee, DRG-S is an effective treatment and that DRG-S was preferred over dorsal column SCS in 83% of patients.
There are currently no RCTs analyzing DRG-S for knee pain, although the observational studies outlined above provide low-quality evidence supporting DRG-S of the L3 and L4 DRG as a treatment option for patients with refractory neuropathic pain confined to the knee.
Foot Pain
Painful conditions of the foot commonly treated with DRG-S include CRPS and phantom limb pain. Sensory innervation for the foot is primarily from the L4, L5, and S1 nerve roots. Foot pain may be difficult to target with traditional dorsal column SCS compared to DRG-S [49]. It is postulated that there is convergence between DRGs at adjacent levels, so placing a lead at one level may result in paresthesias and pain relief at adjacent levels [28, 70]. As outlined above, the ACCURATE trial [6] and another prospective observational study by Liem et al. [5] highlight evidence for DRG-S in treating foot pain.
For chronic foot pain, the most common levels of DRG-S placement are at the L4, L5, and S1 DRGs. In one case of CRPS, recurrence developed after left-sided transtibial amputation and subsequent neuroma excision. Dorsal column SCS was attempted, although the patient did not experience adequate paraesthesia coverage of the stump [35]. DRG-S was subsequently attempted at the left L4 DRG with 75% coverage of stump pain. At 17-month follow-up, the patient reported 60% pain relief with DRG-S. In a case series by Skaribas et al. [28] five patients with CRPS of the foot and prior back surgery underwent S1 DRG-S. The single S1 DRG electrode resulted in pain relief of the entire foot and all five patients achieved substantial improvement in pain intensity and quality of life at 6-month follow-up [28].
Discussion
Summary of Evidence
This review highlights the use of DRG-S for a variety of neuropathic pain syndromes of the lower extremity. The highest level of evidence is demonstrated with DRG-S use for CRPS of the lower extremity based on results from the ACCURATE trial [6]. However, the evidence base overall was downgraded to “low-quality” evidence because of risk of bias, imprecision, heterogeneity, and indirectness from the accompanying observational studies on CRPS. The remaining evidence from observational studies and case series shows promising results for DRG-S in PDN, focal neuropathy, and polyneuropathy, although quality appraisal per the GRADE criteria revealed very low-quality evidence for these indications. DRG-S for refractory postsurgical neuropathic pain in the groin (e.g., post-herniorrhaphy) or in the knee (e.g., post-TKA) has only been demonstrated in small-scale observational studies and future high-quality and adequately powered RCTs are warranted before widespread adoption or recommendation of this practice.
The authors still recommend offering first-line conventional therapy prior to DRG-S or other neuromodulation options. This involves incorporating a multimodal pharmacologic approach for chronic neuropathic pain [71] such as non-steroidal anti-inflammatory agents, gabapentinoids, antidepressants, and other pharmacotherapeutics. If conservative and pharmacologic options fail to provide adequate pain relief, pain specialists may consider offering neuromodulation options including dorsal column SCS, DRG-S, or peripheral nerve stimulation (PNS). The implanting physician may also pursue hybrid neuromodulation trials offering both DRG-S and dorsal column SCS to determine which modality offers better relief prior to implantation.
Unique Features of DRG-S Compared to Other Neuromodulation Interventions
Compared to traditional dorsal column SCS, DRG-S allows for more precise targeting of the DRG that innervates the targeted painful region without nonspecifically stimulating uninvolved dermatomes [4]. Furthermore, DRG-S allows for coverage of groin, pelvic, and foot pain, which is inconsistently treated and is difficult to capture using dorsal column SCS [4]. Finally, because the DRG has a thin layer of cerebrospinal fluid surrounding it, DRG-S maybe achieved with a much lower electrical current and is less impacted by positional changes [4]. Finally, some authors have postulated that analgesia may be achieved in both unstimulated regions and stimulated regions with DRG-S. This involves the concept of “cross-talk”, which involves the convergence between DRGs at adjacent levels, and thus placement of a DRG lead at one level may result in paresthesias and pain relief at adjacent levels [28, 70]. This may also involve contralateral segments due to crossover nerve fibers, as highlighted by a case [49] where unilateral DRG stimulation at the right T12 and S1 resulted in significant pain relief in the bilateral feet and low back. In this case [49], the authors hypothesized that the bilateral pain improvement, despite unilateral lead placement, was due to sympathetic modulation at the DRG with crossover fibers in the sympathetic chain and sacral plexuses. With the advent of PNS for treatment of chronic neuropathic pain syndromes [72], along with a unique profile and mechanism of action [73], implanting physicians may also offer this non-invasive neuromodulation intervention to patients instead of DRG-S. However, in certain more generalized distributions of pain, such as in CRPS which overlaps multiple nerve distributions, DRG-S may be advantageous instead of multiple PNS lead placements.
Proposed Mechanism of Action
The mechanism of analgesia via DRG-S involves reversal of the central pathophysiologic changes seen within the DRG neurons that perpetuate and amplify neuropathic pain [74]. As a response to the initial nerve injury, there is an inflammatory cascade that results from release of excitatory cytokines by immune cells and the supporting satellite glial cells [74]. Furthermore, genetic changes within the DRG neurons also lead to upregulation of ion channels and pain-related receptors and ligands [75], leading to alterations in ion current flux, decreased threshold for action potential firing, and impairment of the DRG’s ability to filter electrical impulses [76]. This mechanism may facilitate ectopic discharges that are relayed to the CNS and interpreted as pain [77].
In a painful state such as CRPS, the filtering across the T-junction of the DRG is decreased [78] along with hyperexcitability in the DRG. The analgesic effects of DRG-S in CRPS are postulated to be through the inhibition of noxious [70, 79, 80] and autonomic [81] afferent signals at the T-junction of the DRG and activation of endogenous opioid system in the dorsal horn [82, 83]. This inhibitory mechanism can attenuate wide-dynamic-range neuronal activity and decrease the windup phenomenon that is observed in CRPS [84].
In vitro and animal studies have also demonstrated that DRG-S can suppress inflammatory responses, even those driven by glial cells [85, 86]. In addition, DRG-S modulates the aberrant ion current flux [52, 87], stabilizes the cell membrane, and restores the DRG’s ability to impede ectopic discharges [79]. Thus, DRG-S normalizes the pathologic hypersensitivity of DRG neurons in neuropathic pain and offers analgesia.
Limitations and Future Directions
This review has several limitations. Clinical and methodological heterogeneity were substantial across included studies. Only one RCT was captured in the search strategy, and the remaining evidence comprised small-scale observational studies and case series/reports. Comparative arms and blinding are difficult to achieve in trials involving DRG-S.
Future studies should compare different waveforms and cost-effectiveness of DRG-S. Comparative studies between DRG-S and PNS for peripheral neuropathy are warranted. Finally, primary care clinicians and neurologists rarely consider DRG-S for treatment of neuropathic pain syndromes in their treatment algorithm. Therefore, dissemination of information and education for both physicians and patients via conference proceedings, social media coverage, and other avenues of information dissemination are important [88].
Conclusion
There is low-quality evidence highlighting that DRG-S is associated with improved pain intensity in lower extremity CRPS. Furthermore, there is very low-quality evidence highlighting that pain relief may be achieved with DRG-S for PDN, focal neuropathy, polyneuropathy, and postsurgical neuropathic pain of the groin and knee. Future high-quality and adequately powered RCTs within more homogeneous participant populations are warranted assessing the utility of DRG-S in lower extremity neuropathic pain syndromes.
References
Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15(10):1669–85. https://doi.org/10.1111/pme.12413.
Hagedorn JM, McArdle I, D’Souza RS, Yadav A, Engle AM, Deer TR. Effect of patient characteristics on clinical outcomes more than 12 months following dorsal root ganglion stimulation implantation: a retrospective review. Neuromodulation. 2021. https://doi.org/10.1111/ner.13326.
Wright RE, Colliton JW. Neurostimulation of the L2 dorsal root ganglion for intractable disc pain: description of a novel technique. Neurosurgery. 1995;36:1101–10.
Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20(Suppl 1):S23–30. https://doi.org/10.1093/pm/pnz012.
Liem L, Russo M, Huygen FJ, et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18(1):41–8. https://doi.org/10.1111/ner.12228 (discussion 48–9).
Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–81. https://doi.org/10.1097/j.pain.0000000000000814.
Deer TR, Pope JE. Dorsal root ganglion stimulation approval by the Food and Drug Administration: advice on evolving the process. Expert Rev Neurother. 2016;16(10):1123–5. https://doi.org/10.1080/14737175.2016.1206817.
Eldabe S, Espinet A, Wahlstedt A, et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation. 2018;21(8):787–92. https://doi.org/10.1111/ner.12767.
Schu S, Gulve A, ElDabe S, et al. Spinal cord stimulation of the dorsal root ganglion for groin pain—a retrospective review. Pain Pract. 2015;15(4):293–9. https://doi.org/10.1111/papr.12194.
Morgalla MH, Bolat A, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation used for the treatment of chronic neuropathic pain in the groin: a single-center study with long-term prospective results in 34 cases. Neuromodulation. 2017;20(8):753–60. https://doi.org/10.1111/ner.12713.
Levy RM, Mekhail N, Kramer J, et al. Therapy habituation at 12 months: spinal cord stimulation versus dorsal root ganglion stimulation for complex regional pain syndrome type I and II. J Pain. 2020;21(3–4):399–408. https://doi.org/10.1016/j.jpain.2019.08.005.
Hagedorn JM, Lam CM, D’Souza RS, et al. Explantation of 10 kHz spinal cord stimulation devices: a retrospective review of 744 patients followed for at least 12 months. Neuromodulation. 2021. https://doi.org/10.1111/ner.13359.
Dombovy-Johnson ML, D’Souza RS, Thuc Ha C, Hagedorn JM. Incidence and risk factors for spinal cord stimulator lead migration with or without loss of efficacy: a retrospective review of 91 consecutive thoracic lead implants. Neuromodulation. 2021. https://doi.org/10.1111/ner.13487.
D’Souza RS, Olatoye OO, Butler CS, Barman RA, Ashmore ZM, Hagedorn JM. Adverse events associated with 10-kHz dorsal column spinal cord stimulation: a 5-year analysis of the manufacturer and user facility device experience (MAUDE) database. Clin J Pain. 2022;38(5):320–7. https://doi.org/10.1097/AJP.0000000000001026.
Hagedorn JM, Romero J, Ha CT, D’Souza RS. Patient satisfaction with spinal cord stimulation and dorsal root ganglion stimulation for chronic intractable pain: a systematic review and meta-analysis. Neuromodulation. 2022. https://doi.org/10.1016/j.neurom.2022.04.043.
Kinfe T, von Willebrand N, Stadlbauer A, et al. Quantitative sensory phenotyping in chronic neuropathic pain patients treated with unilateral L4-dorsal root ganglion stimulation. J Transl Med. 2020;18(1):403. https://doi.org/10.1186/s12967-020-02566-8.
Kavanagh BP. The GRADE system for rating clinical guidelines. PLoS Med. 2009;6(9): e1000094. https://doi.org/10.1371/journal.pmed.1000094.
Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15. https://doi.org/10.1016/j.jclinepi.2010.07.017.
Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. https://doi.org/10.1016/j.jclinepi.2011.03.017.
Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–10. https://doi.org/10.1016/j.jclinepi.2011.04.014.
Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283–93. https://doi.org/10.1016/j.jclinepi.2011.01.012.
Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64(12):1277–82. https://doi.org/10.1016/j.jclinepi.2011.01.011.
Harden NR, Bruehl S, Perez R, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150(2):268–74. https://doi.org/10.1016/j.pain.2010.04.030.
Gravius N, Chaudhry SR, Muhammad S, et al. Selective L4 dorsal root ganglion stimulation evokes pain relief and changes of inflammatory markers: part I profiling of saliva and serum molecular patterns. Neuromodulation. 2019;22(1):44–52. https://doi.org/10.1111/ner.12866.
Huygen F, Liem L, Nijhuis H, Cusack W, Kramer J. Evaluating dorsal root ganglion stimulation in a prospective Dutch cohort. Neuromodulation. 2019;22(1):80–6. https://doi.org/10.1111/ner.12798.
Morgalla MH, de Barros MF, Chander BS, Soekadar SR, Tatagiba M, Lepski G. Neurophysiological effects of dorsal root ganglion stimulation (DRGS) in pain processing at the cortical level. Neuromodulation. 2019;22(1):36–43. https://doi.org/10.1111/ner.12900.
Piedade GS, Vesper J, Chatzikalfas A, Slotty PJ. Cervical and high-thoracic dorsal root ganglion stimulation in chronic neuropathic pain. Neuromodulation. 2019;22(8):951–5. https://doi.org/10.1111/ner.12916.
Skaribas IM, Peccora C, Skaribas E. Single S1 dorsal root ganglia stimulation for intractable complex regional pain syndrome foot pain after lumbar spine surgery: a case series. Neuromodulation. 2019;22(1):101–7. https://doi.org/10.1111/ner.12780.
Van Buyten JP, Smet I, Liem L, Russo M, Huygen F. Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain Pract. 2015;15(3):208–16. https://doi.org/10.1111/papr.12170.
Garg A, Danesh H. Neuromodulation of the cervical dorsal root ganglion for upper extremity complex regional pain syndrome-case report. Neuromodulation. 2015;18(8):765–8. https://doi.org/10.1111/ner.12307.
Pinckard-Dover H, Palmer A, Petersen EA. A review of neuromodulation for treatment of complex regional pain syndrome in pediatric patients and novel use of dorsal root ganglion stimulation in an adolescent patient with 30-month follow-up. Neuromodulation. 2021;24(4):634–8. https://doi.org/10.1111/ner.13257.
van Bussel CM, Stronks DL, Huygen FJ. Successful treatment of intractable complex regional pain syndrome type I of the knee with dorsal root ganglion stimulation: a case report. Neuromodulation. 2015;18(1):58–60. https://doi.org/10.1111/ner.12190 (discussion 60–1).
van Bussel CM, Stronks DL, Huygen FJPM. Dorsal column stimulation vs. dorsal root ganglion stimulation for complex regional pain syndrome confined to the knee: patients’ preference following the trial period. Pain Pract. 2018;18(1):87–93. https://doi.org/10.1111/papr.12573.
Yang A, Hunter CW. Dorsal root ganglion stimulation as a salvage treatment for complex regional pain syndrome refractory to dorsal column spinal cord stimulation: a case series. Neuromodulation. 2017;20(7):703–7. https://doi.org/10.1111/ner.12622.
Goebel A, Lewis S, Phillip R, Sharma M. Dorsal root ganglion stimulation for complex regional pain syndrome (CRPS) recurrence after amputation for CRPS, and failure of conventional spinal cord stimulation. Pain Pract. 2018;18(1):104–8. https://doi.org/10.1111/papr.12582.
Morgalla MH, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation (DRGS) for the treatment of chronic neuropathic pain: a single-center study with long-term prospective results in 62 cases. Pain Physician. 2018;21(4):E377–87.
Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019;22(1):61–79. https://doi.org/10.1111/ner.12796.
Deer TR, Levy RM, Kramer J, et al. Comparison of paresthesia coverage of patient’s pain: dorsal root ganglion vs spinal cord stimulation. An ACCURATE study sub-analysis. Neuromodulation. 2019;22(8):930–6. https://doi.org/10.1111/ner.12920.
Kinfe TM, Asif M, Chakravarthy KV, et al. Unilateral L4-dorsal root ganglion stimulation evokes pain relief in chronic neuropathic postsurgical knee pain and changes of inflammatory markers: part II whole transcriptome profiling. J Transl Med. 2019;17(1):205. https://doi.org/10.1186/s12967-019-1952-x.
Ghosh P, Gungor S. Utilization of concurrent dorsal root ganglion stimulation and dorsal column spinal cord stimulation in complex regional pain syndrome. Neuromodulation. 2021;24(4):769–73. https://doi.org/10.1111/ner.13144.
Pendem K, Jassal N. Dorsal root ganglion stimulation as treatment for complex regional pain syndrome of the foot refractory to spinal cord stimulation: a case report. Cureus. 2021;13(1):e12753. https://doi.org/10.7759/cureus.12753.
Chapman KB, Kloosterman J, Schor JA, Girardi GE, van Helmond N, Yousef TA. Objective improvements in peripheral arterial disease from dorsal root ganglion stimulation: a case series. Ann Vasc Surg. 2021;74:519.e7-519.e16. https://doi.org/10.1016/j.avsg.2021.01.069.
Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93. https://doi.org/10.2337/dc10-1303.
Jensen TS, Backonja MM, Hernández Jiménez S, Tesfaye S, Valensi P, Ziegler D. New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res. 2006;3(2):108–19. https://doi.org/10.3132/dvdr.2006.013.
D’Souza RS, Langford B, Dombovy-Johnson M, Abd-Elsayed A. Neuromodulation interventions for the treatment of painful diabetic neuropathy: a systematic review. Curr Pain Headache Rep. 2022. https://doi.org/10.1007/s11916-022-01035-9.
Hagedorn JM, Engle AM, George TK, et al. An overview of painful diabetic peripheral neuropathy: diagnosis and treatment advancements. Diabetes Res Clin Pract. 2022;188:109928. https://doi.org/10.1016/j.diabres.2022.109928.
D’Souza RS, Barman R, Joseph A, Abd-Elsayed A. Evidence-based treatment of painful diabetic neuropathy: a systematic review. Curr Pain Headache Rep. 2022. https://doi.org/10.1007/s11916-022-01061-7.
Falowski S, Pope JE, Raza A. Early US experience with stimulation of the dorsal root ganglia for the treatment of peripheral neuropathy in the lower extremities: a multicenter retrospective case series. Neuromodulation. 2019;22(1):96–100. https://doi.org/10.1111/ner.12860.
Chapman KB, Van Roosendaal B-KW, Van Helmond N, Yousef TA. Unilateral dorsal root ganglion stimulation lead placement with resolution of bilateral lower extremity symptoms in diabetic peripheral neuropathy. Case reports. Cureus. 2020;12(9):e10735. https://doi.org/10.7759/cureus.10735.
Kretzschmar M, Reining M, Schwarz MA. Three-year outcomes after dorsal root ganglion stimulation in the treatment of neuropathic pain after peripheral nerve injury of upper and lower extremities. Neuromodulation. 2021;24(4):700–7. https://doi.org/10.1111/ner.13222.
Zuidema X, Breel J, Wille F. Paresthesia mapping: a practical workup for successful implantation of the dorsal root ganglion stimulator in refractory groin pain. Neuromodulation. 2014;17(7):665–9. https://doi.org/10.1111/ner.12113 (discussion 669).
Morgalla MH, de Barros Filho MF, Chander BS, Soekadar SR, Tatagiba M, Lepski G. Neurophysiological effects of dorsal root ganglion stimulation (DRGS) in pain processing at the cortical level. Neuromodulation. 2019;22(1):36–43. https://doi.org/10.1111/ner.12900.
Martin SC, Macey AR, Raghu A, et al. Dorsal root ganglion stimulation for the treatment of chronic neuropathic knee pain. World Neurosurg. 2020;11(143):e303–8. https://doi.org/10.1016/j.wneu.2020.07.102.
Hunter CW, Yang A, Davis T. Selective radiofrequency stimulation of the dorsal root ganglion (DRG) as a method for predicting targets for neuromodulation in patients with post amputation pain: a case series. Neuromodulation. 2017;20(7):708–18. https://doi.org/10.1111/ner.12595.
Koetsier E, van Kuijk SMJ, Melli G, et al. Dorsal root ganglion stimulation for the management of intractable painful polyneuropathy: a prospective pilot study. Neuromodulation. 2021;24(4):685–94. https://doi.org/10.1111/ner.13336.
Grabnar M, Kim C. Dorsal root ganglion stimulation for treatment of chemotherapy-induced neuropathy. Case reports journal article. Am J Phys Med Rehabil. 2021;100(4):e52–4. https://doi.org/10.1097/PHM.0000000000001542.
Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation. 2013;16(1):67–71. https://doi.org/10.1111/ner.12013 (discussion 71–2).
Maino P, Koetsier E, Kaelin-Lang A, Gobbi C, Perez R. Efficacious dorsal root ganglion stimulation for painful small fiber neuropathy: a case report. Pain Physician. 2017;20(3):E459–63.
Mata N, Habibi B, Kim C. Dorsal root ganglion stimulation for the treatment of frostbite neuropathy: a case report. Pain Med Case Rep. 2020;4(6):207–10.
Kretzschmar M, Reining M. Dorsal root ganglion stimulation for treatment of central poststroke pain in the lower extremity after medullary infarction. Pain. 2021;162(11):2682–5. https://doi.org/10.1097/j.pain.0000000000002439.
Grabnar M, Kim C. Dorsal root ganglion stimulation for treatment of chemotherapy-induced neuropathy. Am J Phys Med Rehabil. 2021;100(4):e52–4. https://doi.org/10.1097/PHM.0000000000001542.
Roybal AE, Sivanesan E, Chen Y. Case report: Dorsal root ganglion (DRG) stimulation for acute neuropathic pain from acute herpes zoster infection. SAGE Open Med Case Rep. 2021;9:2050313X211062297. https://doi.org/10.1177/2050313X211062297.
Horan M, Jacobsen AH, Scherer C, et al. Complications and effects of dorsal root ganglion stimulation in the treatment of chronic neuropathic pain: a nationwide cohort study in Denmark. Neuromodulation. 2021;24(4):729–37. https://doi.org/10.1111/ner.13171.
Deer TR, Grider JS, Lamer TJ, et al. A systematic literature review of spine neurostimulation therapies for the treatment of pain. Pain Med. 2020;21(7):1421–32. https://doi.org/10.1093/pm/pnz353.
Liem L, Mekhail N. Management of postherniorrhaphy chronic neuropathic groin pain: a role for dorsal root ganglion stimulation. Pain Pract. 2016;16(7):915–23. https://doi.org/10.1111/papr.12424.
Antony AB, Schultheis BC, Jolly SM, Bates D, Hunter CW, Levy RM. Neuromodulation of the dorsal root ganglion for chronic postsurgical pain. Pain Med. 2019;20(Supplement_1):S41–6. https://doi.org/10.1093/pm/pnz072.
Char S, Barman RA, Deer TR, Hagedorn JM. Dorsal root ganglion stimulation for chronic groin pain: a review. Neuromodulation. 2021. https://doi.org/10.1111/ner.13468.
Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019;22(1):61–79. https://doi.org/10.1111/ner.12796.
Martin SC, Macey AR, Raghu A, et al. Dorsal root ganglion stimulation for the treatment of chronic neuropathic knee pain. World Neurosurg. 2020;143:e303–8. https://doi.org/10.1016/j.wneu.2020.07.102.
Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18(1):24–32. https://doi.org/10.1111/ner.12247 (discussion 32).
D’Souza RS, Langford B, Wilson RE, et al. The state-of-the-art pharmacotherapeutic options for the treatment of chronic non-cancer pain. Expert Opin Pharmacother. 2022. https://doi.org/10.1080/14656566.2022.2060741.
Abd-Elsayed A, D’Souza RS. Peripheral nerve stimulation: the evolution in pain medicine. Biomedicines. 2021. https://doi.org/10.3390/biomedicines10010018.
Strand NH, D’Souza R, Wie C, et al. Mechanism of action of peripheral nerve stimulation. Curr Pain Headache Rep. 2021;25(7):47. https://doi.org/10.1007/s11916-021-00962-3.
Znaor L, Lovric S, Hogan Q, Sapunar D. Association of neural inflammation with hyperalgesia following spinal nerve ligation. Croat Med J. 2007;48(1):35–42.
Xiao HS, Huang QH, Zhang FX, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99(12):8360–5. https://doi.org/10.1073/pnas.122231899.
Kovalsky Y, Amir R, Devor M. Simulation in sensory neurons reveals a key role for delayed Na+ current in subthreshold oscillations and ectopic discharge: implications for neuropathic pain. J Neurophysiol. 2009;102(3):1430–42. https://doi.org/10.1152/jn.00005.2009.
Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85(3):503–21. https://doi.org/10.1016/S0304-3959(00)00251-7.
Gemes G, Koopmeiners A, Rigaud M, et al. Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol. 2013;591(4):1111–31. https://doi.org/10.1113/jphysiol.2012.242750.
Kent AR, Min XY, Hogan QH, Kramer JM. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation. 2018;21(3):234–46. https://doi.org/10.1111/ner.12754.
Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain. 2020;161(12):2872–86. https://doi.org/10.1097/j.pain.0000000000001982.
Sverrisdottir YB, Martin SC, Hadjipavlou G, et al. Human dorsal root ganglion stimulation reduces sympathetic outflow and long-term blood pressure. JACC Basic Transl Sci. 2020;5(10):973–85. https://doi.org/10.1016/j.jacbts.2020.07.010.
Chapman KB, Yousef TA, Foster AMDS-H, van Helmond N. Mechanisms for the clinical utility of low-frequency stimulation in neuromodulation of the dorsal root ganglion. Neuromodulation. 2021;24(4):738–45. https://doi.org/10.1111/ner.13323.
Chapman KB, Groenen PS, Vissers KC, van Helmond N, Stanton-Hicks MD. The pathways and processes underlying spinal transmission of low back pain: observations from dorsal root ganglion stimulation treatment. Neuromodulation. 2021;24(4):610–21. https://doi.org/10.1111/ner.13150.
Yang F, Zhang C, Xu Q, et al. Electrical stimulation of dorsal root entry zone attenuates wide-dynamic-range neuronal activity in rats. Neuromodulation. 2015;18(1):33–40. https://doi.org/10.1111/ner.12249 (discussion 40).
Pan B, Zhang Z, Chao D, Hogan QH. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation. 2018;21(3):247–53. https://doi.org/10.1111/ner.12648.
Zhou WT, Ni YQ, Jin ZB, et al. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Muller cells. Exp Neurol. 2012;238(2):192–208. https://doi.org/10.1016/j.expneurol.2012.08.029.
Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation. 2013;16(4):304–11. https://doi.org/10.1111/ner.12028 (discussion 310–1).
Langford B, Hooten WM, D’Souza S, Moeschler S, D’Souza RS. YouTube as a source of medical information about spinal cord stimulation. Neuromodulation. 2020. https://doi.org/10.1111/ner.13303.
Acknowledgements
We would like to acknowledge and thank Larry J. Prokop M.L.S. for assisting with the search strategy.
Funding
No funding was received for this study or the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Ryan S. D’Souza MD helped with study conception and design, performed background of research, analysis, generation of figures, analysis and interpretation of data, and drafted manuscript; Alaa Abd-Elsayed MD helped with study conception and design, performed background of research, drafted portions of the manuscript, revised the manuscript critically for intellectual content, and gave final approval of the manuscript; All authors drafted various portions of the manuscript, revised the manuscript critically for intellectual content, and gave final approval of the manuscript.
Disclosures
Dr. Ryan S. D’Souza has an investigator-initiated grant with Nevro Corp. Dr. Abd-Elsayed is a consultant of Medtronic and Abbott. Other authors have no conflict of interest related to this work. Eva Kubrova, Yeng F. Her, Ross A. Barman DO, Brandon J. Smith, Gabriel M. Alvarez and Tyler E. West have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
D’Souza, R.S., Kubrova, E., Her, Y.F. et al. Dorsal Root Ganglion Stimulation for Lower Extremity Neuropathic Pain Syndromes: An Evidence-Based Literature Review. Adv Ther 39, 4440–4473 (2022). https://doi.org/10.1007/s12325-022-02244-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02244-9