Abstract
Purpose of Review
Autoimmune encephalitis (AE) is an underrecognized and potentially curable disease, which has been the focus of intense neurologic research. In the present manuscript, we review recent updates and the current role of brain positron emission tomography imaging with 18F-fluorodeoxyglucose (FDG-PET) in the detection of AE. We appraise the many metabolic imaging manifestations described in this disease, the role of PET-FDG in its diagnosis and follow-up, and the possible relationship between some patterns and specific autoantibodies. We also briefly discuss recently recognized imaging patterns and the potential impact of new technologies in recognition of such metabolic imaging appearances.
Recent Findings
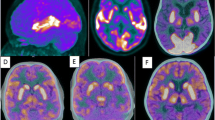
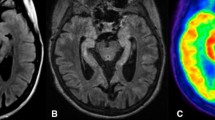
AE findings on FDG-PET may have various patterns, but three are dominant and can be summarized as follows: (1) hypermetabolism in cortical areas, mainly in mesial temporal regions and less frequently in basal ganglia and higher cortical regions, is a common pattern in early stages of the disease. Such pattern is highly suggestive of limbic AE, since it has not been described in many other entities, except for brain tumors and active epileptic foci. (2) Also common is a reduced metabolism in the regions described above, which could happen both in the detection of the disease or in previous hypermetabolic areas which changed their pattern during the course of illness. (3) Other areas with hypometabolism can also occur, especially the “diffuse whole-brain cortical hypometabolism” manifestation, which is unspecific and can have degenerative diseases and other conditions as differential diagnoses. Some antibodies are more related to specific metabolic imaging patterns, but others do not correlate closely with imaging appearances.
Summary
We consider that FDG-PET imaging can aid in the early diagnosis of AE and may also be helpful while accessing the disease longitudinally while showing functional changes that occur after therapy. In both situations it can provide valuable information that is not provided by anatomic imaging alone.





Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McKeon A. Autoimmune encephalopathies and dementias. Contin Lifelong Learn Neurol. 2016;22:538–58.
• Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis—relevance for clinical practice and hippocampal function. Neuroscience 2015; 309:68–83. Well-illustrated update on the state-of-the-art of FDG-PET and AE.
Vollmer T, McCarthy M. Autoimmune encephalitis: a more treatable tragedy if diagnosed early. Neurology. 2016. doi:10.1212/WNL.0000000000002641.
Sekigawa M, Okumura A, Niijima S, Hayashi M, Tanaka K, Shimizu T. Autoimmune focal encephalitis shows marked hypermetabolism on positron emission tomography. J Pediatr. 2010;156:158–60.
Sinmaz N, Amatoury M, Merheb V, Ramanathan S, Dale R, Brilot F. Autoantibodies in movement and psychiatric disorders: updated concepts in detection methods, pathogenicity, and CNS entry. Ann NY Acad Sci. 2015;1351:22–38.
• Baumgartner A, Rauer S, Mader I, Meyer P. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 2013;260:2744–53. One of the studies which tried to correlate imaging appearances with antibodies.
Wingfield T, McHugh C, Vas A, Richardson A, Wilkins E, Bonington A, Varma A. Autoimmune encephalitis: a case series and comprehensive review of the literature. QJM. 2011;104:921–31.
Probasco J, Benavides D, Ciarallo A, Sanin B, Wabulya A, Bergey G, Kaplan P. Electroencephalographic and fluorodeoxyglucose-positron emission tomography correlates in anti-N-methyl-d-aspartate receptor autoimmune encephalitis. Epilepsy Behav Case Rep. 2014;2:174–78.
Höftberger R. Neuroimmunology: an expanding frontier in autoimmunity. Front Immunol. 2015;206:1–6.
Geschwind M. Rapidly progressive dementia. Contin Lifelong Learn Neurol. 2010;16:31–56.
McEvoy LK, Fennema-Notestine C, Roddey JC. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment 1 [Internet]. Radiology. 2009. doi:10.1148/radiol.2511080924.
Fisher R, Patel N, Lai E, Schulz P. Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med. 2012;37:e213–8.
Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz H-G, Peters J, Bartenstein P, Lieb K, Schreckenberger M. SPM-based count normalization provides excellent discrimination of mild Alzheimer’s disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2008;44:43–50.
Catana C, Guimaraes A, Rosen B. PET and MR imaging: the odd couple or a match made in heaven? J Nucl Med. 2013;54:815–24.
Catana C, Drzezga A, Heiss D, Rosen B. PET/MRI for neurologic applications. J Nucl Med. 2012;53:1916–25.
Scheid R, Lincke T, Voltz R, Cramon D, Sabri O. Serial 18F-fluoro-2-deoxy-d-glucose positron emission tomography and magnetic resonance imaging of paraneoplastic limbic encephalitis. Arch Neurol Chic. 2004;61:1785–9.
Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, Dalmau J, Gerloff C, Lewerenz J. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-d-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681–6.
Kumar A. NMDA receptor function during senescence: implication on cognitive performance. Front Neurosci. 2015;9:1–15.
Titulaer M, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig L, Benseler S, Kawachi I, Martinez-Hernandez E, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65.
Dalmau J, Gleichman A, Hughes E, Rossi J, Peng X, Lai M, Dessain S, Rosenfeld M, Balice-Gordon R, Lynch D. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8.
Irani S, Michell A, Lang B, Pettingill P, Waters P, Johnson M, Schott J, Armstrong R, Zagami A, Bleasel A, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900.
Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi J, Voloschin A, Baehring J, Shimazaki H, Koide R, King D, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
Ances B, Vitaliani R, Taylor R, Liebeskind D, Voloschin A, Houghton D, Galetta S, Dichter M, Alavi A, Rosenfeld M, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77.
Chanson J-B, Diaconu M, Honnorat J, Martin T, Seze J, Namer I-J, Hirsch E. PET follow-up in a case of anti-NMDAR encephalitis: arguments for cingulate limbic encephalitis. Epileptic Disord Int Epilepsy J Videotape. 2012;14:90–3.
Lee E, Kang J, Oh J, Kim J, Shin Y-W, Kim C-Y. 18F-fluorodeoxyglucose positron-emission tomography findings with anti-N-methyl-d-aspartate receptor encephalitis that showed variable degrees of catatonia: three cases report. J Epilepsy Res. 2014;4:69–73.
Wegner F, Wilke F, Raab P, Tayeb S, Boeck A-L, Haense C, Trebst C, Voss E, Schrader C, Logemann F, et al. Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-d-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol. 2014;14:136.
Morooka M, Kubota K, Minamimoto R, Furuhata M, Abe T, Ito K, Okasaki M, Ishii K, Ishiwata K. 18F-FDG and 11C-methionine PET/CT findings in a case with anti-NMDA (NR2B) receptor encephalitis. Clin Nucl Med. 2012;37:400–2.
Greiner H, Leach J, Lee K-H, Krueger D. Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure. 2011;20:266–70.
Cistaro A, Caobelli F, Quartuccio N, Fania P, Pagani M. Uncommon 18F-FDG-PET/CT findings in patients affected by limbic encephalitis: hyper–hypometabolic pattern with double antibody positivity and migrating foci of hypermetabolism. Clin Imaging. 2015;39:329–33.
Tobin WO, Strand EA, Clark HM, Lowe VJ. NMDA receptor encephalitis causing reversible caudate changes on MRI and PET imaging. Neurol Clin Pract. 2014;4:470–73.
Yuan J, Guan H, Zhou X, Niu N, Li F, Cui L, Cui R. Changing brain metabolism patterns in patients with ANMDARE: serial 18F-FDG PET/CT findings. Clin Nucl Med. 2016;41:366–70.
Mohr B, Minoshima S. F-18 fluorodeoxyglucose PET/CT findings in a case of anti-NMDA receptor encephalitis. Clin Nucl Med. 2010;35:461–3.
Endres D, Perlov E, Stich O, Rauer S, Maier S, Waldkircher Z, Lange T, Mader I, Meyer P, Elst L. Hypoglutamatergic state is associated with reduced cerebral glucose metabolism in anti-NMDA receptor encephalitis: a case report. BMC Psychiatry. 2015;15:186.
Sonderen A, Schreurs M, Bruijn M, Boukhrissi S, Nagtzaam M, Hulsenboom E, Enting R, Thijs R, Wirtz P, Smitt P, et al. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology. 2016. doi:10.1212/WNL.0000000000002637.
Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P, Psimaras D, Habert M-O, Baulac M, Delattre J-Y, et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain. 2016;139:1079–93.
Shin Y-W, Lee S-T, Shin J-W, Moon J, Lim J-A, Byun J-I, Kim T-J, Lee K-J, Kim Y-S, Park K-I, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol. 2013;265:75–81.
Kamaleshwaran K, Iyer R, Antony J, Radhakrishnan E, Shinto A. 18F-FDG PET/CT findings in voltage-gated potassium channel limbic encephalitis. Clin Nucl Med. 2013;38:392–4.
Park S, Choi H, Cheon G, Kang K, Lee D. 18F-FDG PET/CT in anti-LGI1 encephalitis: initial and follow-up findings. Clin Nucl Med. 2015;40:156–8.
Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–89.
Lai M, Hughes E, Peng X, Zhou L, Gleichman A, Shu H, Matà S, Kremens D, Vitaliani R, Geschwind M, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34.
• Spatola M, Stojanova V, Prior J, Dalmau J, Rossetti A. Serial brain 18FDG-PET in anti-AMPA receptor limbic encephalitis. J Neuroimmunol. 2014;271:53–55. Elegant investigation on anti-AMPA AE and prospective imaging.
Wei Y-C, Liu C-H, Lin J-J, Lin K-J, Huang K-L, Lee T-H, Chang Y-J, Peng T-I, Lin K-L, Chang T-Y, et al. Rapid progression and brain atrophy in anti-AMPA receptor encephalitis. J Neuroimmunol. 2013;261:129–33.
Li X, Mao Y-T, Wu J-J, Li L-X, Chen X-J. Anti-AMPA receptor encephalitis associated with thymomatous myasthenia gravis. J Neuroimmunol. 2015;281:35–7.
Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason W, Kruer M, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–86.
Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen M, Grisold W, Kimura A, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2009;9:67–76.
Su M, Xu D, Tian R. 18F-FDG PET/CT and MRI findings in a patient with anti-GABAB receptor encephalitis. Clin Nucl Med. 2015;40:515–17.
Kim T-J, Lee S-T, Shin J-W, Moon J, Lim J-A, Byun J-I, Shin Y-W, Lee K-J, Jung K-H, Kim Y-S, et al. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol. 2014;270:45–50.
• Lancaster E, Dalmau J. Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–90. Concise and easy to read review on clinical AE pathogenesis.
Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, Ramió-Torrentà L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–63.
Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257:509–17.
Bien C, Vincent A, Barnett M, Becker A, Blümcke I, Graus F, Jellinger K, Reuss D, Ribalta T, Schlegel J, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–38.
Ariño H, Gresa-Arribas N, Blanco Y, Martínez-Hernández E, Sabater L, Petit-Pedrol M, Rouco I, Bataller L, Dalmau J, Saiz A, et al. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71:1009–16.
Rakocevic G, Raju R, Dalakas M. Anti-glutamic acid decarboxylase antibodies in the serum and cerebrospinal fluid of patients with stiff-person syndrome: correlation with clinical severity. Arch Neurol Chic. 2004;61:902–4.
Kojima G, Inaba M, Bruno M. PET-positive extralimbic presentation of anti-glutamic acid decarboxylase antibody-associated encephalitis. Epileptic Disord. 2014;16:358–61.
Malter M, Helmstaedter C, Urbach H, Vincent A, Bien C. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67:470–8.
Acknowledgments
We would like to thank Kimberly Stephens for her kind help with English language issues. AMNC would like to acknowledge the financial support of Sociedade Beneficente Hospital Sirio Libanes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Marianne Kimura Soriano, Carla Rachel Ono, and Artur M. N. Coutinho each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on PET/CT Imaging.
Rights and permissions
About this article
Cite this article
Soriano, M.K., Ono, C.R. & Coutinho, A.M. Positron Emission Tomography with 18F-Fluorodeoxyglucose Imaging Patterns in Autoimmune Encephalitis. Curr Radiol Rep 4, 47 (2016). https://doi.org/10.1007/s40134-016-0174-8
Published:
DOI: https://doi.org/10.1007/s40134-016-0174-8