Abstract
Purpose
F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) is emerging to be a useful tool in supporting the diagnosis of AIE. In this study, we describe the metabolic patterns on F-18 FDG PET imaging in AIE.
Methods
Twenty-four antibody-positive patients (anti-NMDA-15, anti-VGKC/LGI1-6, and anti-GAD-3), 14 females and 10 males, with an age range of 2–83 years were included in this study. Each PET study was evaluated visually for the presence of hypometabolism or hypermetabolism and semiquantitatively using Cortex ID (GE) and Scenium (Siemens) by measuring regional Z-scores. These patterns were correlated with corresponding antibody positivity once available.
Results
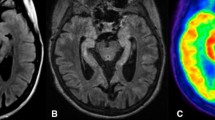
Visually, a pattern of hypometabolism, hypermetabolism, or both in various spatial distributions was appreciated in all 24 patients. On quantitative analysis using scenium parietal and occipital lobes showed significant hypometabolism with median Z-score of −3.8 (R) and −3.7 (L) and −2.2 (R) and −2.5 (L) respectively. Two-thirds (16/24) showed significant hypermetabolism involving the basal ganglia with median Z-score of 2.4 (R) and 3.0 (L). Similarly on Cortex ID, the median Z-score for hypometabolism in parietal and occipital lobes was −2.2 (R) and −2.4 (L) and −2.6 (R) and −2.4 (L) respectively, while subcortical regions were not evaluated. MRI showed signal alterations in only 11 of these patients.
Conclusion
There is heterogeneity in metabolic topography of AIE which is characterized by hypometabolism most commonly involving the parietal and occipital cortices and hypermetabolism most commonly involving the basal ganglia. Scenium analysis using regional Z-scores can complement visual evaluation for demonstration of these metabolic patterns on FDG PET.







Similar content being viewed by others

References
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Dalmau J, Rosenfeld MR (2014) Autoimmune encephalitis update. Neuro-Oncology 61:25–36
Irani SR, Gelfand JM, Bettcher BM, Singhal NS, Geschwind MD (2014) Effect of rituximab in patients with leucine-rich glioma-inactivated 1 antobody associated encephalopathy. JAMA Neurol 71(7):896–900. https://doi.org/10.1001/jamaneurol.2014.463
Baumgartner A, Rauer S, Mader I, Meyer PT (2013) Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 260(260):2744–2753. https://doi.org/10.1007/s00415-013-7048-2
Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A (2010) N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133(6):1655–1667. https://doi.org/10.1093/brain/awq113
Dalmau J, Batallar L (2006) Clinical and immunological diversity of limbic encephalitis: a model for paraneoplastic neuologic disorders. Hematol Oncol Clin North Am 20(6):1319–1335. https://doi.org/10.1016/j.hoc.2006.09.011
Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, Galetta SL, Dichter M, Alavi A, Rosenfeld MR, Dalmau J (2005) Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 128(8):1764–1777. https://doi.org/10.1093/brain/awh526
Scheid R, Lincke T, Voltz R, von Cramon DY, Sabri O (2004) Serial 18F-fluoro-2- deoxy-D-glucose positron emission tomography and magnetic resonance imaging of paraneoplastic limbic encephalitis. Arch Neurol 61(11):1785–1789 2. https://doi.org/10.1001/archneur.61.11.1785
Provenzale JM, Barboriak DP, Coleman RE (1998) Limbic encephalitis: comparison of FDG-PET and MR imaging findings. Am J Roentgenol 170(6):1659–1660 3. https://doi.org/10.2214/ajr.170.6.9609193
Hoffmann LA, Jarius S, Pellkofer HL et al (2008) Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J NeurolNeurosurgPsychia 79:767–773
Blanc F, Ruppert E, Kleitz C, Valenti MP, Cretin B, Humbel RL, Honnorat J, Namer IJ, Hirsch E, Manning L, de Seze J (2009) Acute limbic encephalitis and glutamic acid decarboxylase antibodies: a reality? J Neurol Sci 287(1-2):69–71. https://doi.org/10.1016/j.jns.2009.09.004
Greiner H, Leach JL, Lee KH, Krueger DA (2011) Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure 20(3):266–270. https://doi.org/10.1016/j.seizure.2010.11.013
Pillai SC, Gill D, Webster R et al (2010) Cortical hypometabolism demonstrated by PET in relapsing NMDA receptor encephalitis. PediatrNeurol 43:217–220
Maqbool M, Oleske DA, Huq AH et al (2011) Novel FDG-PET findings in NMDA receptor encephalitis: a case based report. J Child Neurol 26(10):1325–1328 2752. https://doi.org/10.1177/0883073811405199
Caballero PE (2011) Fluorodeoxyglucose positron emission tomography findings in NMDA receptor antibody encephalitis. ArqNeuropsiquiatr 69:409–410 13
Padma S, Sundaram PS, Marmattom BV (2011) PET/CT in the evaluation of anti-NMDA-receptor encephalitis: what we need to know as a NM physician. Ind J Nucl Med 26(2):99–101. https://doi.org/10.4103/0972-3919.90262
Maeder-Ingvar M, Prior JO, Irani SR, Rey V, Vincent A, Rossetti AO (2011) FDG-PET hyperactivity in basal ganglia correlating with clinical course in anti-NDMA-R antibodies encephalitis. J NeurolNeurosurg Psychiatry 82(2):235–236. https://doi.org/10.1136/jnnp.2009.198697
Mohr BC, Minoshima S (2010) F-18 fluorodeoxyglucose PET/CT findings in a case of anti-NMDA receptor encephalitis. ClinNucl Med 35:461–463
Rey C, Koric L, Guedj E, Felician O, Kaphan E, Boucraut J, Ceccaldi M (2012) Striatal hypermetabolism in limbic encephalitis. J Neurol 259(6):1106–1110. https://doi.org/10.1007/s00415-011-6308-2
Dash D, Tripathi M, Ihtisham K, Tripathi M (2016) LGI1 encephalitis: a disease of jerks and confusion. BMJ Case Rep. https://doi.org/10.1136/bcr-2016-217083
Granerod J, Ambrose HE, Davies NW, Clewey JP, Walsh AL, Morgan D et al (2010) Causes of encephalitis and differences in their clinical presentations in England: a multicentre population-based prospective study. Lancet Infect Dis 10(12):835–844. https://doi.org/10.1016/S1473-3099(10)70222-X
Vollmer TL, McCarthy M (2016) Autoimmune encephalitis: a more treatable tragedy if diagnosed early. Neurology 86(18):1655–1656. https://doi.org/10.1212/WNL.0000000000002641
Heine J, Pruss H, Bartsch T, Ploner CJ, Paul F, Finke C (2015) Imaging of autoimmune encephalitis-relevance for clinical practice and hippocampal function. Neuroscience 309:68–83. https://doi.org/10.1016/j.neuroscience.2015.05.037
Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, Dalmau J, Gerloff C, Lewerenz J (2012) Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry 83(7):681–686. https://doi.org/10.1136/jnnp-2011-301969
Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J (2005) Paraneoplastic encephalitis, psychiatric symptoms and hypoventilation in ovarian teratoma. Ann Neurol 58(4):594–604. https://doi.org/10.1002/ana.20614
Fischer RE, Patel NR, Lai EC, Schulz PE (2012) Two different FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med 37(9):e213–e218. https://doi.org/10.1097/RLU.0b013e31824852c7
Wegner F, Wilke F, Raab P, Tayeb S, Boeck A, Haense C et al (2014) Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-D-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in F-18 Fluoro-2-deoxyglucose positron emission tomography. BMC Neurol 14(1):136. https://doi.org/10.1186/1471-2377-14-136
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R (2011) Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 10(1):63–74. https://doi.org/10.1016/S1474-4422(10)70253-2
Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T et al (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12(2):157–165. https://doi.org/10.1016/S1474-4422(12)70310-1
Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, Probasco JC, Javadi MS (2017) Diagnostic value of F-18 FDG PET/CT versus MRI in the setting of antibody specific autoimmune encephalitis. JNM 58(8):1307–1313. https://doi.org/10.2967/jnumed.116.184333
Probasco JC, Solnes LB, Nalluri A, Cohen J, Jones KM, Zan E et al (2017) Abnormal brain metabolism on FDG PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm 4(4):e352. https://doi.org/10.1212/NXI.0000000000000352
Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ (2010) Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 30(17):5866–5875. https://doi.org/10.1523/JNEUROSCI.0167-10.2010
Chakrabarty B, Tripathi M, Gulati S, Yoganathan S, Pandit AK, Sinha A, Rathi BS (2014) Pediatric anti-methyl-D-aspartate (NMDA) receptor encephalitis: experience of a tertiary care teaching centre from north India. J Child Neurol 29(11):1453–1459. https://doi.org/10.1177/0883073813494474
Boesebeck F, Schwarz O, Dohmen B, Graef U, Vestring T, Kramme C, Bien CG (2013) Faciobrachial dystonic seizures arise from cortico-subcortical abnormal brain areas. J Neurol 260(6):1684–1686. https://doi.org/10.1007/s00415-013-6946-7
Fidzinski P, Jarius S, Gaebler C, Boegner F, Nohr R, Ruprecht K (2014) Faciobranchial dystonic seizures and antibodies to LGI1 in a 92-year old patient: a case report. J Neurol Sci 347(1-2):404–405. https://doi.org/10.1016/j.jns.2014.10.026
Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, Schott JM, Armstrong RJE, S. Zagami A, Bleasel A, Somerville ER, Smith SMJ, Vincent A (2011) Faciobranchial dystonic seizures precede LgI1 antibody limbic encephalitis. Ann Neurol 69(5):892–900. https://doi.org/10.1002/ana.22307
Shin Y-W, Lee S-T, Shin J-W, Moon J, Lim J-A, Byun J-I, Kim TJ, Lee KJ, Kim YS, Park KI, Jung KH, Lee SK, Chu K (2013) VGKC complex/LGI1 antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 265(1-2):75–81. https://doi.org/10.1016/j.jneuroim.2013.10.005
Park S, Choi H, Cheon GJ, Wook Kang K, Lee DS (2015) F-18 FDG PET/CT in anti-LGI1 encephalitis: initial and follow-up findings. Clin Nucl Med 40(2):156–158. https://doi.org/10.1097/RLU.0000000000000546
Lancaster E, Dalmau J (2012) Neuronal autoantigens-pathogenesis, associtaed disorders and antibody testing. Nat Rev Neurol 8(7):380–390. https://doi.org/10.1038/nrneurol.2012.99
Saiz A, Blanco Y, Sabater L, Gonzalez F, Bataller L, Casamitijana R et al (2008) Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain 131(10):2553–2563. https://doi.org/10.1093/brain/awn183
Honnorat J, Saiz A, Giometto B, Vincent A, Brieva L, Andreas C et al (2001) Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies. Arch Neurol 58(2):225–230. https://doi.org/10.1001/archneur.58.2.225
Arino H, Gresa-Arribas N, Blaco Y, Martinez-Hernandez E, Sabater L, Petit-Pedrol M et al Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol 71:1009–1016
Newey CR, Sarwal A, Hantus S (2016) F-18 Fluoro-deoxy-glucose positron emission tomography scan should be obtained early in cases of autoimmune encephalitis. Autoimmune Dis 2016:9450452. https://doi.org/10.1155/2016/9450452
Morbelli S, Arbizu J, Booji J, Chen MK, Chetelat G, Cross DJ et al (2017) The need for standardization and of large clinical studies in an emerging indication of F-18 FDG PET: the autoimmune encephalitis. Eur J Nucl Med Mol Imaging 44(3):353–357. https://doi.org/10.1007/s00259-016-3589-9
Acknowledgements
The authors wish to acknowledge Mr. Rajeev Kumar, Medical Physicist, for his help in carrying out the patient studies.
Funding
The antibody profile section of this study was funded by a grant from the Department of Biotechnology (BT/PR-3436/MED/30/651/2011), All India Institute of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tripathi, M., Tripathi, M., Roy, S.G. et al. Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology 60, 189–198 (2018). https://doi.org/10.1007/s00234-017-1956-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1956-2