Abstract
Introduction
The aim of this work is to evaluate the clinical efficacy and safety of sufentanil vs. remifentanil anesthesia in elderly patients undergoing curative resection for hepatocellular carcinoma (HCC).
Methods
Medical records of elderly patients aged ≥ 65 years who received curative resection for HCC between January 2017 and December 2020 were retrospectively reviewed. The patients were divided into either the sufentanil group or the remifentanil group according to the method of analgesia used. Vital signs including mean arterial pressure (MAP), heart rate (HR), and arterial oxygen saturation (SpO2), distribution of T-cell subsets (CD3, CD4, and CD8 lymphocytes), distribution of the stress response index [cortisol (COR), interleukin (IL)-6, C-reactive protein (CRP), and glucose (GLU)] were recorded prior to anesthesia (T0), after induction of anesthesia (T1), at the end of surgery (T2), 24 h after surgery (T3), and 72 h after surgery (T4). Postoperative adverse events were collected.
Results
Repeated measure analysis of variance (ANOVA) showed that after controlling for baseline patient demographic and treatment characteristics as covariates, both between- and within-group effects were significant (all P < 0.01), and the interaction between time and treatments was also significant (all P < 0.01) in the vital signs (MAP, HR, and SpO2), distribution of T-cell subsets (CD3, CD4, and CD8 lymphocytes), and distribution of the stress response index (COR, IL-6, CRP, and GLU), indicating that sufentanil led to stable hemodynamic and respiratory functions, lower reduction of T-lymphocyte subsets, and stable stress response indices compared to remifentanil. There is no significant difference in adverse reactions between the two groups (P = 0.72).
Conclusions
Sufentanil was associated with improved hemodynamic and respiratory function, less stress response, less inhibition of cellular immunity, and similar adverse reactions compared with remifentanil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related mortality worldwide. Induction of anesthesia is critical for the prognosis of the HCC operation. |
Sufentanil and remifentanil are widely adopted in many operations. However, few comparative studies are available on the application of the two agonists in the surgical treatment of HCC. |
What was learned from the study? |
Sufentanil was associated with improved hemodynamic and respiratory function, less stress response, less inhibition of cellular immunity, and similar adverse reactions compared to remifentanil. |
Sufentanil has the potential to become the first choice of anesthesia to be used in the clinic for elderly patients who undergo curative resection for HCC. |
Introduction
Hepatocellular carcinoma (HCC) is a common cancer in patients with chronic liver disease and is the leading cause of cancer-related mortality worldwide. According to the World Health Organization, HCC is responsible for over 800,000 new cases and an estimated 783,000 deaths in 2018 [1]. China is a region with a high incidence of liver cancer, and more than 53% of the total HCC patients in the world come from China [2].
Surgical treatment has become the most widely used and effective method for the treatment of HCC [3]. In the HCC operation, induction of anesthesia is an important activity and is commonly associated with high chances of hemodynamic instability, hypoxia, arrhythmias, and excitatory reflexes. Thus, the induction process should be quick and devoid of any such effects [4]. Sufentanil and remifentanil are opioid receptor agonists with great analgesic effects and good safety profiles in many operations [5,6,7,8]. However, few comparative studies are available on the application of the two agonists in the surgical treatment of HCC.
This study aims to evaluate the clinical efficacy and safety of sufentanil vs. remifentanil anesthesia in elderly patients undergoing surgery for HCC.
Methods
Patients
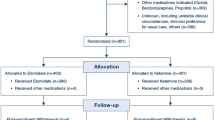
Elderly patients (≥ 65 years) who received curative resection for HCC, with administrating sufentanil or remifentanil at our hospital from January 2017 to December 2020 were included in this study. The inclusion criteria were as follows: postoperative pathology shows hepatocellular carcinoma, diagnosis of primary liver cancer stage I–III according to the eighth edition of American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging, complete clinical basic information, perioperative laboratory test results, and postoperative follow-up data. The exclusion criteria included preoperative chemotherapy and radiotherapy, preoperative infectious diseases, autoimmune diseases, and other malignant tumors, loss of follow-up, and lack of perioperative hematology records. The patients were divided into either the sufentanil group or the remifentanil group according to the method of analgesia used.
This study was approved by the Ethics Committee of the General Hospital of Central Theater Command of the Chinese People's Liberation Army (approval No. 2022013). All procedures involving human participants were performed by the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. As this is a retrospective study, informed consent was waived by the Ethic Committee of the General Hospital of Central Theater Command of the Chinese People's Liberation Army.
Anesthesia Care
Before surgery, an electrocardiogram and monitoring of vital signs were implemented in the two groups of patients. In the sufentanil group, patients were induced by a bolus of midazolam, 0.07 mg/kg (Nhwa Pharmaceutical Co., Ltd, Xuzhou, China), and a bolus of sufentanil, 2 μg/kg (Humanwell Healthcare; Yichang, China). Mechanical ventilation was carried out when the plasma concentration of patients was in a state of equilibrium. During surgery, a continuous infusion of sufentanil (0.025 μg/kg/min) was started. Additional midazolam was given as an intravenous bolus, 0.03–0.07 mg/kg, as indicated by the anesthesiologist. In the remifentanil group, induction was achieved using a continuous infusion of remifentanil, 1 μg/kg/min. Ventilation and usage of midazolam were performed as in the sufentanil group. The injection of sufentanil or remifentanil ceased at the end of surgery. Temperature-holding nursing was implemented for the two groups. After patients regained their respiratory function and consciousness, catheters were removed, and patients were sent to the recovery room.
Data Collection
Vital signs including mean arterial pressure (MAP), heart rate (HR), arterial oxygen saturation (SpO2), distribution of T-cell subsets (CD3, CD4, and CD8 lymphocytes), and distribution of the stress response index [cortisol (COR), interleukin (IL)-6, C-reactive protein (CRP), and glucose (GLU)] were recorded prior to (T0) and after (T1) anesthesia, at the end of surgery (T2), 24 h after surgery (T3), 72 h after surgery (T4). Postoperative adverse events were collected.
Statistical Analysis
Continuous variables with normal distribution are presented as means ± SD and compared with the use of Student’s t test. All categorical variables were summarized and expressed as proportions and compared with the use of the chi-square test or Fisher’s exact test, as appropriate. Repeated Measure analysis of variance (ANOVA) was used for the comparison of the data at different time points, and LSD post hoc test was used. All tests were two sided and a P value of less than 0.05 was considered significant.
All statistical analyses were performed with the SPSS statistical software program package (SPSS version 20.0 for Windows, Armonk, NY: IBM Corp.).
Results
Patients’ Characteristics
Of 529 patients undergoing curative resection for HCC from January 2017 to December 2020, 460 patients met the inclusion and exclusion criteria and were assigned to the sufentanil group (n = 322) or remifentanil group (n = 138). As shown in Table 1, baseline patient demographic and treatment characteristics including age, gender, American Society of Anesthesiologists (ASA) grade, surgical procedure, surgical type, anesthesia time, liver cirrhosis, preoperative alpha-fetoprotein (AFP), TNM staging, tumor size, tumor number, vascular invasion, estimated blood loss and intraoperative blood transfusion did not differ significantly between the sufentanil and remifentanil regimens.
Comparison of Hemodynamic Parameters Between Sufentanil and Remifentanil Groups
Repeated measure ANOVA showed that after controlling for baseline patient demographic and treatment characteristics as covariates, both between- and within-group effects were significant (both P < 0.01), and the interaction between time and treatments was also significant (P < 0.01) in the MAP, HR, and SpO2 (Fig. 1), indicating that sufentanil led to stable hemodynamic and respiratory functions compared to remifentanil during the follow-up. Post hoc analysis showed that the differences between the two groups in the MAP, HR, and SpO2 were significant (P < 0.01) after induction of anesthesia (T1) and at the end of surgery (T2), and not significant (P > 0.05) prior to anesthesia (T0), 24 h after surgery (T3), and 72 h after surgery (T4).
Comparison of T-cell Subsets Between Sufentanil and Remifentanil Groups
As shown in Fig. 2, after controlling for baseline patient demographic and treatment characteristics as covariates, repeated measure ANOVA showed that both between- and within-group effects were significant (both P < 0.01), and the interaction between time and treatments was also significant (P < 0.01) in the CD3, CD4, and CD8. Post hoc analysis showed that the differences between the two groups in the CD3, CD4, and CD8 were not significant (P > 0.05) prior to anesthesia (T0), but statistically significant (P < 0.01) from after induction of anesthesia (T1) to 72 h after surgery (T4), indicating that the degree of reduction of T-lymphocyte subsets in the sufentanil group was lower than that in the remifentanil group.
Comparison of Stress Response Indices Between Sufentanil and Remifentanil Groups
Repeated measure ANOVA showed that after controlling for baseline patient demographic and treatment characteristics as covariates, both between- and within-group effects were significant (both P < 0.01), and the interaction between time and treatments was also significant (P < 0.01) in the COR, IL-6, CRP, and GLU (Fig. 3). Post hoc analysis showed that the differences between two groups in the COR, IL-6, CRP, and GLU were not significant (P > 0.05) prior to anesthesia (T0), but statistically significant (P < 0.01) from after induction of anesthesia (T1) to 72 h after surgery (T4), indicating that stress response indices in the sufentanil group maintained stable and required a shorter recovery following surgery.
Changes in the distribution of the stress response index including cortisol (COR, A), interleukin (IL)-6 (B), C-reactive protein (CRP, C), and glucose (GLU, D) in the sufentanil group and remifentanil group. T0 prior to anesthesia, T1 after anesthesia, T2 at the end of surgery, T3 24 h after surgery, T4 72 h after surgery
Comparison of Adverse Reactions Between Sufentanil and Remifentanil Groups
As shown in Table 2, difference in the rate of the adverse reaction between sufentanil group and the remifentanil group was not statistically significant (P = 0.72).
Discussion
In this retrospective study, we compared the clinical efficacy and safety of sufentanil vs. remifentanil anesthesia in patients undergoing surgery for HCC. The results indicated that sufentanil led to stable hemodynamic and respiratory functions, lower reduction of T-lymphocyte subsets, stable stress response indices, and similar adverse reactions compared to remifentanil.
Vital signs are of great importance for surgical anesthesia. Our results indicated that in the remifentanil group, there were a significant increase in HR and significant decreases in MAP and SpO2 at T1 and T2 compared with those before surgery (P < 0.05). In contrast, changes in the HR, SpO2, and MAP from T0–T5 were not obvious in the sufentanil group. Results from a study comparing recovery effect for sufentanil and remifentanil anesthesia with TCI in laparoscopic radical resection during colorectal cancer were consistent with our findings [5]. Thus, sufentanil-based anesthesia is demonstrated to maintain stable vital signs and bring less influence on respiratory function and hemodynamics indices.
Curative resection for HCC leads to stress response in patients [9, 10]. The stress response is a non-specific defense reaction to external stimulation. Previous studies have indicated that stress responses may be initiated by anesthesia and surgery, and influence post-surgery recovery [5, 11, 12]. Blood COR and GLU could reflect the intensity of stress response, and IL-6 is one of the important cytokines that are involved in the inflammatory response and injury repair and reflect the degree of human stress [13]. Our results indicated that concentrations of GLU, COR, IL-6, and CRP significantly increased during anesthesia and operation in patients treated with remifentanil group and required a longer time to recover after operation. In contrast, these concentrations were stable in the sufentanil group, indicating that sufentanil can maintain stable blood GLU and COR with much less stress response.
Owing to T lymphocytes and their subsets’ capacity for antigen-directed cytotoxicity, they have become a central focus for engaging the immune system in the fight against cancer. CD3 is expressed on the surface of mature T lymphocytes, and CD4 helper/inducer cells and CD8 cytotoxic/suppressor cells are two phenotypes of T lymphocytes, characterized by distinct surface markers and functions that mostly reside in lymph nodes but also circulate in the blood [14, 15]. CD3 could recognize abnormal cells by binding molecules called antigens, CD4 promotes B-cells to further differentiate and produce antibodies, while CD8 inhibits T-cell proliferation. In our study, reductions of CD3, CD4, and CD8 cells in the sufentanil group were substantially lower compared to those in the remifentanil group, with a rapid recovery of cellular immune function.
Our study has several limitations. First, owing to the retrospective nature, residual confounding from unknown and unmeasured variables is possible. Second, relatively few patients were exposed to remifentanil, and the inference on the effect of remifentanil is weakened. Finally, this study was conducted exclusively based on a research center from a homogenous healthcare system, which may limit the generalization.
Conclusions
In conclusion, sufentanil was associated with improved hemodynamic and respiratory function, less stress response, less inhibition of cellular immunity, and similar adverse reactions compared with remifentanil.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Wu Q, Qin SK. Features and treatment options of Chinese hepatocellular carcinoma. Chin Clin Oncol. 2013;2(4):38.
Lee MW, Lim HK. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: current status and future. World J Gastroenterol. 2018;24(46):5215–22.
Dhande K, Kshirsagar J, Dhande A, Patil N, V P, Jr. Hemodynamic Stability, Patient Acceptance and Cost of Intravenous Propofol and Inhalational Sevoflurane for Induction of Anaesthesia: A Prospective, Randomized Comparative Study. Cureus. 2020;12(4):e7687.
Qi Y, Yao X, Zhang B, Du X. Comparison of recovery effect for sufentanil and remifentanil anesthesia with TCI in laparoscopic radical resection during colorectal cancer. Oncol Lett. 2016;11(5):3361–5.
Zhao R, Xu X, Sun L, Zhang G. Long-term effect of anesthesia choice on patients with hepatocellular carcinoma undergoing open liver resection. Front Oncol. 2022;12: 960299.
Huang JY, Shih PC, Chen CT, Lin HY, Chien YJ, Wu MY, et al. Effects of short-acting opioids on intraocular pressure during general anesthesia: systematic review and network meta-analysis. Pharmaceuticals (Basel). 2022;15(8):989.
Zhu Y, Wang Y, Du B, Xi X. Could remifentanil reduce duration of mechanical ventilation in comparison with other opioids for mechanically ventilated patients? A systematic review and meta-analysis. Crit Care. 2017;21(1):206.
Ueda K, Turner P, Gagner M. Stress response to laparoscopic liver resection. HPB (Oxford). 2004;6(4):247–52.
Kasai M, Van Damme N, Berardi G, Geboes K, Laurent S, Troisi RI. The inflammatory response to stress and angiogenesis in liver resection for colorectal liver metastases: a randomized controlled trial comparing open versus laparoscopic approach. Acta Chir Belg. 2018;118(3):172–80.
Cabanero D, Puig MM. Immediate and delayed remifentanil-induced hypersensitivity. Anesth Analg. 2012;115(4):977–8; author reply 8–9.
Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64(4):301–7.
Gonzalez-Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Horm Behav. 2002;41(3):328–33.
Yalcin N, Uzun ST, Reisli R, Borazan H, Otelcioglu S. A comparison of ketamine and paracetamol for preventing remifentanil induced hyperalgesia in patients undergoing total abdominal hysterectomy. Int J Med Sci. 2012;9(5):327–33.
Bi SS, Deng CH, Zhou TY, Guan Z, Li L, Li HQ, et al. Remifentanil-sevoflurane interaction models of circulatory response to laryngoscopy and circulatory depression. Br J Anaesth. 2013;110(5):729–40.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
NJ and FL conceived the study idea and designed the study; NJ, YZ, LL, JD and ST collected the data; NJ and LL performed the data analysis; NJ drafted the manuscript and FL made critical revision. All authors reviewed the manuscript and agreed to submit it.
Disclosures
The authors have no conflicts of interest to declare.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of the General Hospital of Central Theater Command of the Chinese People's Liberation Army (approval No. 2022013). All procedures involving human participants were performed by the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. As this is a retrospective study, informed consent was waived by the Ethics Committee of the General Hospital of Central Theater Command of the Chinese People's Liberation Army.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available, as it could compromise the privacy of research participants, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ji, N., Zhang, Y., Li, L. et al. Comparison of Recovery Effect for Sufentanil vs. Remifentanil Anesthesia in Elderly Patients Undergoing Curative Resection for Hepatocellular Carcinoma. Pain Ther 12, 1017–1025 (2023). https://doi.org/10.1007/s40122-023-00522-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00522-0