Abstract
Introduction
The outcomes of transfemoral (TF) compared with transapical (TA) access for transcatheter aortic valve replacement (TAVR) in diabetics are unknown.
Methods
We queried the NIS database (2011–2014) to identify diabetics who underwent TAVR. We performed a propensity matching analysis comparing TF-TAVR versus TA-TAVR.
Results
The analysis included 14.555 diabetics who underwent TAVR. After matching, in-hospital mortality was not different between TF-TAVR and TA-TAVR. (3.5 vs. 4.4%, p = 0.11). TF-TAVR was associated with lower rates of cardiogenic shock (2.7 vs. 4.7%, p = 0.02), use of mechanical circulatory support (2.0 vs. 2.9%, p = 0.03), acute renal failure (17.8 vs. 26.5%, p < 0.001), major bleeding (35.8 vs. 40.7%, p < 0.001) and respiratory complications (1.1 vs. 4.4%, p < 0.001) compared with TA-TAVR. However, TF-TAVR was associated with a higher rate of vascular complications (2.9 vs. 0.9%, p < 0.001), cardiac tamponade (0.5 vs. 0.0%, p < 0.001), complete heart block (10.8 vs. 7.7%, p < 0.001) and pacemaker insertion (11.8 vs. 8.3%, p < 0.001). There was no difference between both groups in acute stroke (1.8 vs. 2.2%, p = 0.39), hemodialysis (2.0 vs. 2.2%, p = 0.71), and ventricular arrhythmias (4.9 vs. 4.2%, p = 0.19). Notably, TF-TAVR was associated with higher mortality, acute stroke, AKI, hemodialysis, PCI, and respiratory complications in complicated diabetics compared with non-complicated diabetics.
Conclusions
This observational analysis showed no difference in-hospital mortality between TF-TAVR and TA-TAVR among diabetic patients. Studies exploring the optimal access for TAVR among diabetics are recommended.
Similar content being viewed by others
There is a paucity of data on the comparative outcomes between trans-femoral and trans-apical accesses in diabetics undergoing TAVR. |
We found no overall difference among diabetics between TF-TAVR and TA-TAVR as regards to in-hospital mortality. |
Compared with TA-TAVR, TF-TAVR was associated with lower rates of cardiogenic shock, major bleeding, respiratory complications, and shorter length of stay, at the expense of higher incidence of vascular complications, cardiac tamponade, and permanent pacemaker requirements with TF-TAVR. |
Subgroup analysis demonstrated that TF-TAVR was associated with higher mortality among complicated diabetics, and lower mortality among non-complicated diabetics, compared with TA-TAVR. |
Introduction
Transcatheter aortic valve replacement (TAVR) has become a viable alternative compared with surgical aortic valve replacement (SAVR) in patients with aortic stenosis irrespective of the surgical risk [1,2,3]. Diabetic patients with aortic stenosis have a different disease profile compared to the general population, with more rapid disease progression and tendency toward left ventricular remodeling and dysfunction [4, 5]. Studies have proposed TAVR as an appealing option for diabetics, compared with SAVR [6], yet its outcomes remain affected by the burden of diabetes mellitus (DM) [5]. Among TAVR patients, DM was demonstrated as an independent predictor of short- and long-term mortality in patients undergoing TAVR [7,8,9]. DM is a known risk factor for microvascular and macrovascular angiopathies [5]. Also, diabetics were found to have higher rates of vascular complications post-TAVR [10]. In this study, we hypothesized that there is an interaction between the access site of TAVR procedures and the outcomes among diabetics undergoing TAVR. To evaluate this hypothesis, we conducted an observational analysis using real-world data to compare outcomes of transapical (TA) versus transfemoral (TF) TAVR procedures among diabetic patients.
Methods
The data source for this study was the National Inpatient Sample (NIS) database. The NIS is the largest inpatient all-payer healthcare database. Unweighted, the NIS contains data from more than 7 million hospital stays each year, while after appropriate weighting, it estimates more than 35 million hospital stays nationally. The NIS was developed for the Health Care Cost and Utilization Project (HCUP) [11]. The NIS has been validated internally and externally [12, 13]. It has been used previously for describing trends and outcomes of various diseases [14, 15]. This study was exempt from local institutional review board approval, since the NIS contains de-identified data that are publicly available.
We queried the NIS years 2011–2014 to identify hospitalizations that have the International Classification of Diseases, Ninth Edition (ICD-9) procedure codes for TAVR procedures (trans-femoral 35.05 and trans-apical 35.06). We then selected records that carried ICD-9 clinical modification codes for DM (ICD-9-CM codes: 250.00 to 250.33 and 250.40 to 250.93). We excluded cases with missing data on comorbidities, in-hospital mortality, or other study outcomes.
We conducted a propensity-score matched analysis to compare hospitalizations with DM who underwent TF-TAVR to those who underwent TA-TAVR. We reported the trends of TA-TAVR and TF-TAVR in diabetic patients during the study years. The main outcome was all-cause in-hospital mortality. Other outcomes included: cardiogenic shock, acute myocardial infarction (MI), cardiac tamponade, acute stroke, acute kidney injury (AKI), hemodialysis for AKI, major bleeding, requirements of blood transfusion, vascular complications, ventricular arrhythmias, complete heart block, use of mechanical circulatory support devices (MCS), permanent pacemaker insertions, length of hospital stay, and discharges to skilled nursing facilities. Baseline characteristics and clinical outcomes were reported using relevant ICD-9 codes, CCS, and Elixhauser comorbidities as reported by HCUP (Supplemental Table 1).
We conducted a 1:1 propensity score analysis to match TF-TAVR with TA-TAVR, using MatchIt R package. X [16]. Nearest-neighbor technique was adopted to match each case to a control that is closest in terms of calculated propensity score. The propensity score was calculated from the following clinical variables: age, sex, race, hypothyroidism, fluid/electrolytes abnormalities, hypertension, liver disease, heart failure, history of smoking, chronic kidney disease (CKD), chronic lung disease, peripheral arterial disease (PAD), anemia, pulmonary circulatory disorders, obesity, history of percutaneous coronary intervention (PCI), previous coronary artery bypass grafting (CABG), and prior MI. Prespecified subgroup analyses were conducted for all study outcomes in TF-TAVR versus TA-TAVR in patients with complicated DM compared with those with uncomplicated DM. Complicated DM was defined per DM-related complications including neuropathy, nephropathy, ophthalmopathy, and angiopathies. In the subgroup analysis, to maintain the baseline balance between the TF-TAVR and TA-TAVR groups, only the corresponding matched pairs in a subgroup were selected.
We used the updated weighting samples for national estimates in accordance with HCUP regulations [17]. We compared categorical values using Chi-square test and continuous variables using Student’s t test. We reported categorical variables as numbers and percentages, while continuous variables were reported as mean \( \pm \) standard deviation or median and interquartile range, depending on the skewness of distribution. Breslow–Day test was used to test the homogeneity of the odds ratio. Linear regression analysis was used to evaluate time trend analyses. Effect sizes were expressed using odds ratios (OR) and 95% confidence interval (CI). Associations were considered significant if the p value was < 0.05. We used SPSS software (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY, USA: IBM Corp Released 2016) and R software for all statistical analysis [18].
Results
The study flow sheet is outlined in Fig. 1. From 2011 to 2014, our search yielded 14,555 diabetics who underwent TAVR. After excluding 12 cases with missing baseline characteristics, a total of 14,543 hospitalizations were included. TF-TAVR was performed in 11,769 (80.9%) of those hospitalizations, while TA-TAVR was performed in 2774 (19.1%) hospitalizations. There was no change in the trend of TF-TAVR or TA-TAVR procedures in diabetics from 2011 to 2014 (Ptrend = 0.60 and 0.41, respectively) (Fig. 2). Propensity score analysis, the matched cohort included a total of 5437 hospitalizations; 2718 in the TF-TAVR and 2719 in the TA-TAVR groups.
The baseline characteristics of the study population are outlined in Table 1. Before matching, the TF-TAVR group were more likely to be older, females, whites, African Americans, and to have a history of heart failure, prior PCI, CKD, pulmonary circulation disorders, obesity, and anemia. The TA-TAVR group had higher prevalence of Hispanics, Asians, prior CABG, chronic lung disease, and PAD. After matching, the standardized mean differences between both groups in the baseline characteristics were all less than 10% suggesting minimal differences (Supplemental Fig. 1).
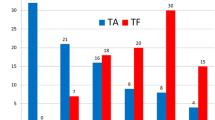
After matching, in-hospital mortality was not different between TF-TAVR and TA-TAVR (3.5 vs. 4.4%, OR 0.79; 95% CI 0.60–1.04, p = 0.11). TF-TAVR was associated with lower rates of cardiogenic shock (2.7 vs. 4.7%, OR 0.61; 95% CI 0.46–0.82, p = 0.02), utilization of MCS (2.0 vs. 2.9%, OR 0.67; 95% CI 0.47–0.95, p = 0.03), AKI (17.8 vs. 26.5%, OR 0.60; 95% CI 0.53–0.68, p < 0.001), major bleeding (35.8 vs. 40.7%, OR 0.82; 95% CI 0.73–0.91, p < 0.001), blood transfusions (21.4 vs. 31.3%, OR 0.60; 95% CI 0.53–0.68, p < 0.001), respiratory complications (1.1 vs. 4.4%, OR 0.24; 95% CI 0.16–0.36, p < 0.001), discharge to skilled facilities (26.1 vs. 39.3%, OR 0.55; 95% CI 0.49–0.61, p < 0.001), and shorter mean length of stay (7.8 ± 6.8 vs. 9.9 ± 7.4 days, p < 0.001) compared with TA-TAVR. However, TF-TAVR was associated with a higher rate of vascular complications (2.9 vs. 0.9%, OR 3.4; 95% CI 2.1–5.3, p < 0.001), cardiac tamponade (0.5 vs. 0.0%, OR 0.1.005; 95% CI 1.002–1.008, p < 0.001), complete heart block (10.8 vs. 7.7%, OR 1.45; 95% CI 1.20–1.75, p < 0.001) and permanent pacemaker insertion (11.8 vs. 8.3%, OR 1.49; 95% CI 1.25–1.78, p < 0.001). There was no difference between both groups in acute stroke (1.8 vs. 2.2%, OR 0.83; 95% CI 0.57–1.2, p = 0.39), acute MI (2.6 vs. 2.8%, OR 0.93; 95% CI 0.67–1.30, p = 0.74) hemodialysis (2.0 vs. 2.2%, OR 0.92; 95% CI 0.63–1.33, p = 0.71) and ventricular arrhythmias (4.9 vs. 4.2%, OR 1.19; 95% CI 0.92–1.53, p = 0.19) (Fig. 3) (Table 2).
On subgroup analysis, TF-TAVR in patients with complicated diabetes was associated with higher rate of in-hospital mortality compared with TA-TAVR (7.7 vs. 2.0%, OR 4.13; 95% CI 2.02–8.44, p < 0.001), while in non-complicated diabetics TF-TAVR was associated with lower in-hospital mortality compared with TA-TAVR (2.7 vs. 4.9%, OR 0.53; 95% CI 0.38–0.73, p < 0.001); Pinteraction < 0.001. Results of subgroup analysis for the other study outcomes are presented in Table 3. Compared with non-complicated diabetics, TF-TAVR among complicated diabetics was associated with higher rate of acute stroke (Pinteraction = 0.05), AKI (Pinteraction = 0.05), hemodialysis (Pinteraction = 0.05), blood transfusions (Pinteraction = 0.03), percutaneous coronary intervention (Pinteraction = 0.02), and respiratory complications (Pinteraction < 0.001).
Discussion
In this observational analysis including 14,543 hospitalizations, we sought to evaluate the comparative outcomes between trans-femoral and trans-apical accesses in diabetics undergoing TAVR. After propensity matching, we found no overall difference among diabetics between TF-TAVR and TA-TAVR as regards to in-hospital mortality. After matching, TF-TAVR was associated with lower rates of cardiogenic shock, utilization of MCS, AKI, major bleeding, blood transfusions, respiratory complications, and shorter length of stay compared with TA-TAVR. On the other side, TF-TAVR was associated with higher incidence of vascular complications, cardiac tamponade, complete heart block, and permanent pacemaker requirements. No difference was observed between both groups in the rates of acute stroke, acute MI, hemodialysis, and ventricular arrhythmias. Subgroup analysis showed that among complicated diabetics, TF-TAVR was associated with higher rates of in-hospital mortality, acute stroke, AKI, hemodialysis, PCI, and respiratory complications, compared with non-complicated diabetics.
Diabetes is a traditional risk factor that has been established to confer additional morbidity and mortality to various surgical and transcatheter procedures [19, 20]. DM is included as a risk factor in the Society of Thoracic Surgeons Risk Score and EuroSCORE II, both of which are validated tools in predicting 30-day mortality after cardiac surgery [19, 20]. Specifically, studies have suggested an interaction for diabetes with clinical outcomes after TAVR, with reports of unfavorable outcomes associating diabetics undergoing TAVR at short and long term [7,8,9]. Abramowitz et al. conducted an analysis using the Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/TVT) Registry, including 47,643 patients. Their analysis showed that diabetes was a significant predictor of 1-year mortality [5]. Also, in the Ibero-American registry including 1220 TAVRs, diabetes was found to be an independent predictor of long-term mortality [7]. However, the impact of access site on the interaction between diabetes and TAVR procedures has not been adequately characterized.
Multiple studies have compared TF-TAVR and TA-TAVR in all comers with results suggesting favorable short- and long-term mortality with TF-TAVR. Kumar et al. conducted an analysis using the NIS database to compare TF-TAVR and TA-TAVR in all comers. Their results showed that TF-TAVR was associated with lower rates of in-hospital mortality compared with TA-TAVR [21]. Results from other registries showed similar survival benefit with TF-TAVR [22, 23].
Unlike the studies on all-comers, our analysis showed no significant difference between trans-femoral and trans-apical accesses in diabetics undergoing TAVR. This lack of difference might be attributed to higher incidences of vascular complications and bradyarrhythmia complications, which might have neutralized the overall benefits observed with TF-TAVR in studies on all-comers. In our analysis, we found a threefold higher rates of vascular complications among diabetics who underwent TF-TAVR compared with TA-TAVR. Prior studies have demonstrated that diabetes is associated with higher vascular complications among patients undergoing TF-TAVR [10, 24]. In a pooled analysis from the Placement of Aortic Transcatheter Valve (PARTNER) Trial, major vascular complications were evaluated in 419 patients [10]. Insulin-dependent diabetics had more than threefold higher rates of vascular complications compared with non-diabetics [10].
Consistent with other studies, TF-TAVR had more favorable hemodynamic outcomes; with less rates of cardiogenic shock, use of MCS, AKI, as well as less respiratory complications [25, 26]. We found no difference between TF-TAVR and TA-TAVR in acute stroke. The theoretical benefit for TA-TAVR by avoiding manipulation of the aorta and direct valve implantation, did not translate to lower stroke risk in many clinical studies, similar to our results [25, 27].
In our study, subgroup analysis identified significant interaction between the status of diabetes (i.e., complicated or not) with mortality outcomes in TF-TAVR compared with TA-TAVR. Such interaction seemed to be driven by higher rates of acute stroke, AKI, hemodialysis, and PCI for TF-TAVR among complicated diabetics. Patients with complicated diabetes are mostly insulin-dependent and are likely to have diabetes-related complications. Other reports have suggested an interaction between the status of diabetes and outcomes after TAVR. In the analysis by Abramowitz et al., insulin-treated diabetes was a stronger predictor of 1-year mortality compared with non-insulin-treated diabetes among TAVR patients [5], driven by higher requirements of hemodialysis, MI, and heart failure readmissions [5]. Data from an Italian registry showed that being insulin-treated DM, but not orally treated DM, was an independent predictor of mortality and MI at 1-year follow-up [9]. Data from a single-center study also showed the same results with worse mid-term mortality after TAVR in insulin-treated diabetics [28].
Compared to all-comers undergoing TAVR, the relatively worse outcomes with DM, in particularly complicated DM, could be related to the pathophysiological changes associating DM. Diabetic patients with severe aortic stenosis have a different profile compared with the general population. They have more accelerated progression of AS, left ventricular remodeling, and reduced systolic function compared with non-diabetic AS patients [4, 5]. The worse outcomes with advanced DM are attributed to diabetes-related complications including renal disease and vasculopathies at multiple vascular beds with more propensity for cardiac, cerebrovascular, and peripheral vascular complications. Increased post-operative inflammation and oxidative stress among diabetics is also a contributing factor to worse post-procedural outcomes [29].
This current analysis is the first analysis to date exploring the impact of DM on access site for TAVRs. The lack of mortality benefit with TF-TAVR versus TA-TAVR in diabetics compared with studies on all-comers is an important finding. Patients with complicated DM might have higher in-hospital mortality with TF-TAVR compared with TA-TAVR. The results of our subgroup analysis highlight the importance of careful patient selection and individualized decisions on access sites for TAVR in diabetic patients. Further studies are warranted to explore the outcomes of alternative access sites for TAVR in diabetics, in particular complicated diabetes.
This analysis has several limitations. The NIS is an administrative database, which is liable to coding and documentation errors. It is also a time-discrete database, with no available data on long-term outcomes. Given the timeframe of our study, the evaluated TAVR procedures were mostly using first-generation TAVR valves. Newer generations of TAVR valves have smaller vascular profiles and might carry less vascular complications. Also, the use of TA-TAVR has decreased and has lower incidence than that reported in our study. Other relevant information could not be retrievable from this dataset including data on imaging tests, types of TAVR valves utilized, or laboratory results. Being an observational analysis, there is potential for selection bias. However, we conducted a propensity match analysis to reduce allocation biases. Nevertheless, the possibility of unmeasured confounders exists. Despite the aforementioned limitations, the current study contributes to the literature regarding the impact of diabetes on outcomes of TAVR procedures.
Conclusions
This observational analysis of a large national database showed no difference in in-hospital mortality between TF-TAVR and TA-TAVR among diabetic patients. Among complicated diabetics, TF-TAVR might be associated with unfavorable outcomes compared with TA-TAVR. Studies exploring the optimal access for TAVR among diabetics are still required.
References
Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–95.
Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696–704.
Elgendy IY, Mahmoud AN, Gad MM, Elbadawi A, Rivero F, Alfonso F. Transcatheter or Surgical Aortic Valve Replacement for Low Surgical Risk Patients: Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2019;12:1399–401.
Nakamura T, Toda K, Kuratani T, Miyagawa S, Yoshikawa Y, Fukushima S, et al. Diabetes mellitus impairs left ventricular mass regression after surgical or transcatheter aortic valve replacement for severe aortic stenosis. Heart Lung Circ. 2016;25(1):68–74.
Abramowitz Y, Vemulapalli S, Chakravarty T, Li Z, Kapadia S, Holmes D, et al. Clinical impact of diabetes mellitus on outcomes after transcatheter aortic valve replacement: insights from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2017;10(11):e005417.
Lindman BR, Pibarot P, Arnold SV, Suri RM, McAndrew TC, Maniar HS, et al. Transcatheter versus surgical aortic valve replacement in patients with diabetes and severe aortic stenosis at high risk for surgery: an analysis of the PARTNER Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol. 2014;63(11):1090–9.
Muñoz-García AJ, del Valle R, Trillo-Nouche R, Elízaga J, Gimeno F, Hernández-Antolín R, et al. The Ibero-American transcatheter aortic valve implantation registry with the CoreValve prosthesis. Early and long-term results. Int J Cardiol. 2013;169(5):359–65.
Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308.
Conrotto F, D’Ascenzo F, Giordana F, Salizzoni S, Tamburino C, Tarantini G, et al. Impact of diabetes mellitus on early and midterm outcomes after transcatheter aortic valve implantation (from a multicenter registry). Am J Cardiol. 2014;113(3):529–34.
Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol. 2012;60(12):1043–52.
Elixhauser A, McCarthy E. Clinical classifications for health policy research, version 2: Hospital inpatient statistics: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1996.
Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305(17):1769–76.
Mahmoud AN, Taduru SS, Mentias A, Mahtta D, Barakat AF, Saad M, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv. 2018;11(1):80–90.
Elbadawi A, Elgendy IY, Mentias A, Ogunbayo GO, Tahir MW, Biniwale N, et al. National trends and outcomes of endomyocardial biopsy for patients with myocarditis: from the National Inpatient Sample database. J Card Fail. 2018;24(5):337–41.
Elbadawi A, Elgendy IY, Mahmoud K, Lenka J, Olorunfemi O, Reyes A, et al. National trends and outcomes of percutaneous coronary intervention in patients ≥ 70 years of age with acute coronary syndrome (from the National Inpatient Sample database). Am J Cardiol. 2018;123:25–32.
Ho DE, Imai K, King G, Stuart E. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2006;42(8):1–28.
Houchens R, Ross D, Elixhauser A. Using the HCUP National Inpatient Sample to estimate trends. 2015. HCUP Methods Series Report# 2006-05 ONLINE. http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Published January 4, 2016. Accessed 27 Mar 2017.
Team RC. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2015. http://www.R-project.org. Accessed 25 June 2015.
Shahian DM, O’brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thoracic Surg. 2009;88(1):S2–22.
Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. Euroscore ii. Eur J Cardiothorac Surg. 2012;41(4):734–45.
Kumar N, Khera R, Fonarow GC, Bhatt DL. Comparison of outcomes of transfemoral versus transapical approach for transcatheter aortic valve implantation. Am J Cardiol. 2018;122(9):1520–6.
Ludman P, Moat N, de Belder M, Blackman D, Duncan A, Banya W, et al. UK TAVI Steering Committee and the National Institute for Cardiovascular Outcomes Research. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation. 2015;131(13):1181–90.
Blackstone EH, Suri RM, Rajeswaran J, Babaliaros V, Douglas PS, Fearon WF, et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation. 2015;131(22):1989–2000.
Kadakia MB, Herrmann HC, Desai ND, Fox Z, Ogbara J, Anwaruddin S, et al. Factors associated with vascular complications in patients undergoing balloon-expandable transfemoral transcatheter aortic valve replacement via open versus percutaneous approaches. Circ Cardiovasc Interv. 2014;7:570–6.
Kumar N, Khera R, Fonarow GC, Bhatt DL. Comparison of outcomes of transfemoral versus transapical approach for transcatheter aortic valve implantation. Am J Cardiol. 2018;122:1520–6.
Arbel Y, Zivkovic N, Mehta D, Radhakrishnan S, Fremes SE, Rezaei E, et al. Factors associated with length of stay following trans-catheter aortic valve replacement-a multicenter study. BMC Cardiovasc Disord. 2017;17(1):137.
Athappan G, Gajulapalli RD, Sengodan P, Bhardwaj A, Ellis SG, Svensson L, et al. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a meta-analysis and systematic review of literature. J Am Coll Cardiol. 2014;63(20):2101–10.
Abramowitz Y, Jilaihawi H, Chakravarty T, Mangat G, Maeno Y, Kazuno Y, et al. Impact of diabetes mellitus on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2016;117(10):1636–42.
Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart. 2015;101(7):537–45.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
An abstract for this manuscript was presented at the Society for Cardiovascular Angiography & Interventions (SCAI) annual meeting May 2019, Las Vegas, NV, USA.
Disclosures
Ayman Elbadawi, Ahmed H. Mohamed, Mohamed A. Omer, Islam Y. Elgendy, Ahmed Abuzaid, Gbolahan Ogunbayo, Michael Megaly, Hend I Shahin, Karim Mahmoud, Ken Fujise, and Syed Gilani have nothing to disclose.
Compliance with Ethics Guidelines
This study was exempt from local institutional review board, since the NIS contains de-identified data that are publicly available.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10008341.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Elbadawi, A., Mohamed, A.H., Elgendy, I.Y. et al. Comparative Outcomes of Transapical Versus Transfemoral Access for Transcatheter Aortic Valve Replacement in Diabetics. Cardiol Ther 9, 107–118 (2020). https://doi.org/10.1007/s40119-019-00155-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-019-00155-5