Abstract
Purpose
Candidemia is associated with high mortality especially in critically ill patients. Our aim was to identify predictors of mortality among critically ill patients with candidemia with a focus on early interventions that can improve prognosis.
Methods
Multicenter retrospective study.
Setting
This retrospective study was conducted in Intensive Care Units from three European university hospitals from 2015 to 2021. Adult patients with at least one positive blood culture for Candida spp. were included. Patients who did not require source control were excluded. Primary outcome was 14-day mortality.
Results
A total of 409 episodes of candidemia were included. Most candidemias were catheter related (173; 41%), followed by unknown origin (170; 40%). Septic shock developed in 43% episodes. Overall, 14-day mortality rate was 29%. In Cox proportional hazards regression model, septic shock (P 0.001; HR 2.20, CI 1.38–3.50), SOFA score ≥ 10 points (P 0.008; HR 1.83, CI 1.18–2.86), and prior SARS-CoV-2 infection (P 0.003; HR 1.87, CI 1.23–2.85) were associated with 14-day mortality, while combined early appropriate antifungal treatment and source control (P < 0.001; HR 0.15, CI 0.08–0.28), and early source control without appropriate antifungal treatment (P < 0.001; HR 0.23, CI 0.12–0.47) were associated with better survival compared to those without neither early appropriate antifungal treatment nor source control.
Conclusion
Early source control was associated with better outcome among candidemic critically ill patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections caused by Candida spp. are frequently encountered among patients admitted to Intensive Care Units (ICU). According to the second Extended Prevalence of Infection in Intensive Care (EPIC II) study, Candida spp accounted for 19% of all infections in Europe [1]. Over recent decades, the epidemiology of candidemia has undergone a significant shift characterized by an increase in its incidence and a rise in the prevalence of C. non-albicans species [2, 3]. In Europe, C. albicans still remains the most commonly isolated species in central or northern countries, while C. parapsilosis has become predominant in southern regions [2, 3]. The incidence of candidemia also saw a rise in some countries during the Coronavirus Disease 2019 (COVID-19) pandemic [4,5,6].
Candidemia poses a significant threat to critically ill patients and is associated with high mortality rates, especially among those with septic shock [3, 7, 8]. Adequate and timely antifungal treatment plays a pivotal role in patient survival, with echinocandins being recommended as the preferred choice according to current guidelines [8,9,10,11,12,13,14]. In addition, prompt source control, like catheter removal in catheter-related candidemia or drainage of abscesses in intra-abdominal candidiasis, has shown varying results in reducing mortality in previous studies [11,12,13,14,15,16,17,18,19]. However, the combined effect of these interventions (antifungal treatment, source control) on mortality has not been extensively explored [7, 11,12,13, 17, 20, 21].
In a prior study conducted at Lausanne University Hospital, the impact of source control, particularly catheter removal, on candidemia outcomes in patients with sepsis or septic shock, was demonstrated [20]. To further investigate the significance of timely source control and identify other potential predictors of mortality, our multicenter study aimed to validate these findings, focusing on critically ill patients with candidemia across two European countries, Greece and Switzerland.
Materials and methods
Study design
This retrospective multicenter study was conducted during a 7-year period (2015–2021) at three tertiary hospitals: the University General Hospital of Patras (UGHP) and the University General Hospital of Heraklion (UGHH) in Greece, and the Lausanne University Hospital (LUH) in Switzerland.
Patients
Inclusion criteria included: adult patients (≥ 18 years old), at least one positive blood culture set for a Candida spp., and admission in ICU within 48 h from candidemia onset. Patients who did not require source control were excluded. The primary outcome was 14-day mortality, and the secondary one was 30-day mortality. Data on demographics, comorbidities, septic shock, antifungal treatment, source control procedures, decisions regarding care withdrawal, and outcomes were collected. Infectious diseases specialists conducted daily rounds in all three ICUs during the study period.
Candida species were identified by Vitek-2 YST card (bioMerieux, Marcy l’Etoile, France) in UGHP and UGHH and by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (Bruker, Billerica, MA) in LUH. Antifungal susceptibility testing in UGHP and UGHH was performed by Etest (bioMérieux) on RPMI-2% glucose agar, and by microbroth dilution method (Sensititre YeastOneTM, Trek Diagnostics Systems, ThermoFisher Scientific, Cleveland, OH) in LUH. Results of minimal inhibitory concentrations (MIC) were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) clinical breakpoints [22]. Beta-d-glucan was available in the LUH since 2017.
Definitions
Candidemia onset was defined as the date the first positive blood culture was drawn. We regarded a new episode to have occurred when more than 30 days had passed since the first negative blood culture from the initial episode. Septic shock followed the Sepsis-3 International Consensus definition [23]. Catheter-related candidemia was defined per IDSA guidelines, either by a positive catheter tip culture showing the same organism as in the candidemia (across all hospitals), or by a positive differential time to positivity favoring the blood culture drawn from the catheter (only in LUH) [24]. In all three hospitals, catheter insertion was guided by echography, and specific protocols were in place to address catheter-related infections. Appropriate antifungal treatment was defined as administrating an antifungal agent, for which the isolate was defined as susceptible according to CLSI criteria [22], at an adequate dosage and diffusion in the infection site. Source control was warranted for catheter-related candidemia (removal of all intravascular catheters), candidemia of unknown origin (removal of all intravascular catheters) intra-abdominal infection (surgical or imaging-guided drainage of abscess, peritoneal collection), obstructive urinary-tract infection (removal of obstruction), endocarditis (valvular replacement). We used the cutoff of 72 h to define early interventions (antifungal treatment initiation, source control) from candidemia onset, which corresponded to the usual time to positivity of Candida spp. in blood cultures [20]. Patients were considered to be on maximal care until a decision of treatment withdrawal or instauration of palliative care has been documented in the medical record.
Statistical analyses
Data analysis utilized SPSS version 26.0 (SPSS, Chicago, IL, USA). Categorical variables were analyzed with Chi-square or Fisher exact test and continuous variables with Mann–Whitney U test for 14-day and 30-day mortality as the dependent variables. Covariates were tested for multi-collinearity through variance inflation factor assessment; those with P < 0.1 in the univariate analysis and not collinear were used in multivariate analysis. After checking Cox assumptions, two multivariate Cox proportional hazards regression models were performed with 14- and 30-day mortality as the time-to-event. Hazzard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association. All statistic tests were two-tailed and P < 0.05 was considered statistically significant.
Kaplan–Meier curves of the survival probability of patients with candidemia that survived for at least 72 h according to early appropriate source control and early appropriate antifungal treatment were performed, with patients being divided in four groups:
-
Group 1: neither early source control nor early appropriate antifungal treatment
-
Group 2: only early appropriate antifungal treatment
-
Group 3: only early source control
-
Group 4: early source control and early appropriate antifungal treatment
Kaplan–Meier curves of the survival probability were performed in the subgroups of patients with candidemia of unknown origin, catheter-related candidemia, presence of septic shock. Since it was previously suggested that source control could be influenced by care withdrawal [25], Kaplan–Meier curves were performed among patients that were alive and in maximal care for 7 days after candidemia onset to assess the role of early source control on survival.
Results
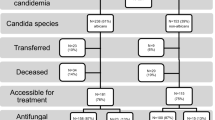
Of the 443 identified candidemia episodes, 409 episodes in 382 patients met the inclusion criteria (UGHP: 226, UGHH: 92, LUH: 91) (Fig. 1). A total of 414 Candida strains were isolated (2 different species were isolated in 5 episodes). C. parapsilosis was identified as the most prevalent species (181; 44%), followed by C. albicans (119; 29%), C. glabrata (57; 14%) and C. tropicalis (41; 10%) (Table 1). Sixteen isolates (4%) belonged to other Candida spp. C. parapsilosis was the most common species isolated in UGHP (60%) and UGHH (45%), while C. albicans predominated in LUH (60%). According to CLSI criteria, 181 (44%) isolates showed resistance or dose-dependent susceptibility to fluconazole (UGHP: 61%, UGHH: 22%, LUH: 26%), 35 (9%) were resistant or intermediate to at least one echinocandin (UGHP: 14%, UGHH: 1%, LUH: 3%), and 6 (2%) to amphotericin B (UGHP: 1%, UGHH: 0%, LUH: 3%). In the LUH, beta-D-glucan was performed in 28 (30%) episodes and was positive in 27 (96%). Eighteen episodes (4%) were acquired in other hospitals departments within 48 h from ICU admission.
Most candidemia episodes were catheter related (173; 42%), followed by unknown origin (170; 42%) and secondary to intra-abdominal infection (37; 9%). Septic shock was present in 173 episodes (42%). One-hundred (52%) episodes occurred in patients with SARS-CoV-2 infection (UGHP: 79; 63% UGHH: 16; 39%, LUH: 5; 21%).
Antifungal treatment was initiated early in 340 (83%) episodes (UGHP: 84%, UGHH: 86%, LUH: 78%) and it was appropriate in 322 (79%) episodes (UGHP: 79%, UGHH: 82%, LUH: 75%). Source control was performed in 370 episodes (90%), with 317 (78%) of them being performed early.
The 14-day, 30-day, and overall ICU mortality rates were 29%, 43%, and 62%, respectively. Antifungal treatment was administered in 390 (95%) episodes, with 340 (93%) receiving antifungal treatment within 72 h of candidemia onset; of these, 322 (95%) were considered appropriate. Care withdrawal within the first 7 days from candidemia onset was decided in 13 (3%) episodes. Table 2 displays the comparison of the characteristics of candidemia episodes between patients who survived and those who deceased within 14 days. In Cox proportional hazards regression model (Table 3), septic shock (P 0.001; HR 2.20, CI 1.38–3.50), SOFA score ≥ 10 points (P 0.008; HR 1.83, CI 1.18–2.86), and prior SARS-CoV-2 infection (P 0.003; HR 1.87, CI 1.23–2.85) were associated with 14-day mortality. On the other hand, the combination of early appropriate antifungal treatment and source control (P < 0.001; HR 0.15, CI 0.08–0.28), early source control without early appropriate antifungal treatment (P < 0.001; HR 0.23, CI 0.12–0.47) were associated with improved survival compared to those who received neither early appropriate antifungal treatment nor source control.
Supplementary Table 1 shows the comparison of the characteristics of candidemia episodes between patients who survived and those who deceased within 30 days. In Cox proportional hazards regression model (Supplementary Table 2), female sex (P 0.040; HR 1.39, CI 1.02–0.90), septic shock (P 0.031; HR 1.47, CI 1.04–2.07), SOFA score ≥ 10 points (P < 0.001; HR 2.00, CI 1.43–2.80), and prior SARS-CoV-2 infection (P 0.006; HR 1.65, CI 1.16–2.35) were associated with 30-day mortality. On the other hand, the combination of early appropriate antifungal treatment and source control (P < 0.001; HR 0.12, CI 0.07–0.21), early source control without early appropriate antifungal treatment (P < 0.001; HR 0.27, CI 0.18–0.39) were associated with improved survival at day 30 compared to those who received neither early appropriate antifungal treatment nor source control.
Figure 2 shows Kaplan–Meier curves illustrating the survival probability of episodes with candidemia based on early appropriate antifungal treatment and early source control in the 390 cases that survived at least 72 h from candidemia onset. Patients in Group 2 (those who received only early appropriate antifungal treatment) exhibited a similar outcome (P 0.120) to those in Group 1 (individuals who received neither early appropriate antifungal treatment nor early source control). However, both Groups 1 and 2 experienced worse outcomes (P < 0.001) compared to Groups 3 (patients with only early source control) and 4 (individuals who received both early appropriate antifungal treatment and early source control). There was no significant difference observed when comparing Groups 3 and 4.
Kaplan–Meier curves illustrating the survival probability of episodes with candidemia based on early appropriate antifungal treatment and early source control in the 390 cases that survived at least 72 h from candidemia onset. Patients in Group 2 (those who received only early appropriate antifungal treatment) exhibited a similar outcome (P 0.120) to those in Group 1 (individuals who received neither early appropriate antifungal treatment nor early source control). However, both Groups 1 and 2 experienced worse outcomes (P < 0.001) compared to Groups 3 (patients with only early source control) and 4 (individuals who received both early appropriate antifungal treatment and early source control). There was no significant difference observed when comparing Groups 3 and 4
Supplemental Fig. 1 presents Kaplan–Meier curves illustrating the survival probabilities of patients with candidemia in the following scenarios: (A) patients who remained under maximal care for 7 days following the onset of candidemia, (B) patients with candidemia of unknown origin, (C) patients with catheter-related candidemia, and (D) patients with septic shock.
Discussion
The present study aimed to investigate predictors of mortality among candidemic patients requiring source control in three university hospitals, representing two regions with distinct epidemiological profiles, susceptibility patterns, and clinical management practices. Our findings underscore the paramount importance of source control, which emerged as the most influential factor affecting patient outcomes.
Consistent with previous publications, our study underscores the benefits of timely source control interventions, particularly catheter removal, in enhancing patient survival [11,12,13,14,15, 20]. This aligns with guidelines recommending early source control, although the debate surrounding its efficacy continues, driven by varying study outcomes and constraints in conducting randomized controlled trials [7, 9, 11,12,13,14,15, 18, 25,26,27,28,29]. One notable challenge is that catheter removal is not always feasible or safe, especially in cases of severe thrombocytopenia, administration of vasoactive drugs, or continuous renal replacement therapy. For instance, in a randomized trial on candidemia, only 51% of patients underwent early catheter removal, despite it being protocol recommended [30]. Therefore, retrospective observational studies such as ours, influenced by patient-specific factors, maximal care versus palliative approaches, and infection severity, face limitations in drawing definitive conclusions [25, 31, 32]. When considering patients on maximal care versus those with care withdrawal, it becomes evident that the absence of source control due to care withdrawal plays a significant role [7, 19, 25, 31]. To address this, we performed Kaplan–Meier curves among patients who remained alive and under maximal care for 7 days after candidemia onset, reaffirming the significant impact of source control on survival. It is worth noting that the participating hospitals exhibited heterogeneous management strategies, with Greek hospitals (UGHP: 87% and UGHH: 80%) more frequently performing early source control compared to LUH (51%). This disparity may be attributed to higher rates of catheter-related bloodstream infections in Greek ICUs [33]. In addition, the prevalence of C. parapsilosis, commonly associated with catheter-related candidemia, was higher in Greek ICUs than in LUH. Although C. parapsilosis was previously associated with a better outcome, the species of Candida species did not exert any influence on mortality in the present study [34].
In the majority of patients (83%) an antifungal therapy was initiated early (within 72 h from candidemia onset), and was considered appropriate in 79% of them. Interestingly, contrary to previous findings, the initiation of appropriate antifungal therapy was not associated with survival in our study [8, 10,11,12,13]. This may be attributed to the more pronounced impact of source control on outcomes. Previous studies have highlighted the role of empiric antifungal choice in patient outcomes, with echinocandins being associated with reduced mortality, especially in patients with septic shock [8, 11, 35]. However, in our study, 24% of critically ill patients received initial antifungal therapy with non-echinocandin drugs (fluconazole or liposomal amphotericin B), and this did not lead to worse outcomes compared to the group initially receiving echinocandin therapy.
While most studies have demonstrated the favorable impact of either prompt source control or early appropriate antifungal treatment on outcomes, not all have evaluated the significance of early combined management and which component is more crucial [7, 11,12,13, 17, 20, 21]. Previous studies have indicated that a combination of early source control and early appropriate antifungal treatment is associated with improved outcomes. In contrast, considering each intervention separately (i.e., source control or early appropriate antifungal therapy) has not consistently shown significant associations [7, 14]. Bassetti et al. demonstrated that both inadequate source control and inadequate antifungal therapy were individual predictors of worse outcomes [21]. In the present study, patients receiving both early source control and appropriate antifungal treatment exhibited comparable survival to those with only early source control. In addition, early appropriate antifungal treatment was not associated with a better outcome when compared to those without both early source control and appropriate antifungal treatment. These findings underscore the paramount importance of prompt source control in managing critically ill candidemic patients.
In the present study, SARS-CoV-2 infection was associated with increased mortality, aligning with prior reports [4, 6]. We observed a lower 30-day mortality among COVID-19 candidemic patients (36%) compared to previously reported rates (60–88%) [4, 6, 36]. An increase in incidence of candidemia among critically ill COVID-19 patients has been reported in the literature [4,5,6], which was more prominent in UGHP among the participating hospitals. This increased incidence may be attributed to factors such as the higher administration of immunosuppressive treatments (e.g., corticosteroids and tocilizumab) and broad-spectrum antibiotics among COVID-19 patients [36, 37].
As previously demonstrated, infection severity, as indicated by the SOFA score or the development of septic shock, was associated with mortality [10, 11, 14, 16, 17, 27]. Early source control was significantly associated with better outcome in patients with septic shock and those without. This was also shown in two previous studies with ICU candidemic patients with septic shock [7, 21]. Unlike previous research, host-related factors such as advanced age or comorbidities did not influence outcome [7, 11, 13, 15, 17].
The present study has several limitations. First, it is a retrospective study; however, it included a high number of critically ill patients from three university centers each with its distinct incidence rates, epidemiology, and clinical management practices. Second, the use of a 72-h cutoff for defining early source control and antifungal treatment may appear arbitrary. As shown in a study from LUH, approximately 30% of candidemias became positive in blood cultures after 72 h [20]. Moreover, cultures positive before 72 h faced delays in pathogen identification due to working hours, subsequently impacting source control and antifungal treatment initiation. In addition, the two Greek ICUs did not have access to rapid diagnostic tests [38]. Although beta-d-glucan was available at LUH, its usage was infrequent and, as previously demonstrated, was employed to either refrain from or discontinue empirical antifungal therapy [39]. Furthermore, no data on hydroalcoholic consumption, site of intravascular catheter insertion (jugular, subclavian, or femoral), and type of disinfection were available. Lastly, no research was conducted on the virulence or biofilm formation of different Candida spp.
In conclusion, this multicenter study conducted in the ICU of three university centers with varying epidemiological and clinical practices underscores the critical importance of prompt source control, particularly catheter removal in cases of catheter-related candidemia or candidemia of unknown origin. Hence, for patients diagnosed with candidemia, in addition to promptly initiating appropriate antifungal treatment, it is imperative to expeditiously undertake source control procedures, an aspect that is often overlooked in clinical practice.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9.
Goemaere B, Becker P, Van Wijngaerden E, et al. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses. 2018;61:127–33.
Montagna MT, Lovero G, Borghi E, et al. Candidemia in intensive care unit: a nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur Rev Med Pharmacol Sci. 2014;18:661–74.
Kayaaslan B, Eser F, Kaya Kalem A, et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64:1083–91.
Papadimitriou-Olivgeris M, Kolonitsiou F, Kefala S, et al. Increased incidence of candidemia in critically ill patients during the Coronavirus disease 2019 (COVID-19) pandemic. Braz J Infect Dis. 2022;26: 102353.
Seagle EE, Jackson BR, Lockhart SR, Georgacopoulos O, Nunnally NS, Roland J, Barter DM, Johnston HL, Czaja CA, Kayalioglu H, Lockhart SR, et al. The landscape of candidemia during the Coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis. 2022;74:802–11.
Kollef M, Micek S, Hampton N, et al. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739–46.
Papadimitriou-Olivgeris M, Spiliopoulou A, Fligou F, et al. Risk factors and predictors of mortality of candidaemia among critically ill patients: role of antifungal prophylaxis in its development and in selection of non-albicans species. Infection. 2017;45:651–7.
Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–5.
Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–22.
Labelle AJ, Micek ST, Roubinian N, et al. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. 2008;36:2967–72.
Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, et al. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother. 2013;68:206–13.
Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect. 2014;20:O245-254.
Horn DL, Ostrosky-Zeichner L, Morris MI, et al. Factors related to survival and treatment success in invasive candidiasis or candidemia: a pooled analysis of two large, prospective, micafungin trials. Eur J Clin Microbiol Infect Dis. 2010;29:223–9.
Kutlu M, Sayin-Kutlu S, Alp-Cavus S, et al. Mortality-associated factors of candidemia: a multi-center prospective cohort in Turkey. Eur J Clin Microbiol Infect Dis. 2022;41:597–607.
Ohki S, Shime N, Kosaka T, et al. Impact of host- and early treatment-related factors on mortality in ICU patients with candidemia: a bicentric retrospective observational study. J Intensive Care. 2020;8:30.
Nucci M, Anaissie E, Betts RF, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis. 2010;51:295–303.
Nucci M, Braga PR, Nouer SA, et al. Time of catheter removal in candidemia and mortality. Braz J Infect Dis. 2018;22:455–61.
Papadimitriou-Olivgeris M, Battistolo J, Poissy J, et al. Key role of early source control in candidemic patients with sepsis or septic shock. Open Forum Infect Dis. 2022;9:ofac383.
Bassetti M, Righi E, Ansaldi F, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40:839–45.
Clinical and Laboratory Standards Institute (CLSI) (2022) Performance standards for antifungal susceptibility testing of yeasts, 3rd ed. CLSI supplement M27M44S. Clinical and Laboratory Standards Institute, Wayne
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.
Damonti L, Erard V, Garbino J, et al. Catheter retention as a consequence rather than a cause of unfavorable outcome in candidemia. Intensive Care Med. 2017;43:935–9.
Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18:19–37.
Lee YM, Kim DY, Kim YJ, et al. Clinical impacts of delayed central venous catheter removal according to the severity of comorbidities in patients with candidaemia. J Hosp Infect. 2019;103:420–7.
Liu CY, Huang LJ, Wang WS, et al. Candidemia in cancer patients: impact of early removal of non-tunneled central venous catheters on outcome. J Infect. 2009;58:154–60.
Cortes JA, Montanez AM, Carreno-Gutierrez AM, et al. Risk factors for mortality in colombian patients with candidemia. J Fungi (Basel). 2021;7:442.
Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–27.
Rodriguez D, Park BJ, Almirante B, et al. Impact of early central venous catheter removal on outcome in patients with candidaemia. Clin Microbiol Infect. 2007;13:788–93.
Janum S, Afshari A. Central venous catheter (CVC) removal for patients of all ages with candidaemia. Cochrane Database Syst Rev. 2016;7:CD011195.
European Centre for Disease Prevention and Control (ECDC). Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013.
Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74:323–31.
Kato H, Hagihara M, Shibata Y, et al. Comparison of mortality between echinocandins and polyenes for an initial treatment of candidemia: a systematic review and meta-analysis. Infect Chemother. 2021;27:1562–70.
Omrani AS, Koleri J, Ben Abid F, et al. Clinical characteristics and risk factors for COVID-19-associated candidemia. Med Mycol. 2021;59:1262–6.
Riche CVW, Cassol R, Pasqualotto AC. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J Fungi (Basel). 2020;6:286.
Papadimitriou-Olivgeris M, Andrianaki AM, Marangos M, et al. Hospital-wide antifungal prescription in Greek hospitals: a multicenter repeated point-prevalence study. Eur J Clin Microbiol Infect Dis. 2020;39:243–8.
Kritikos A, Poissy J, Croxatto A, et al. Impact of the Beta-Glucan Test on management of Intensive Care Unit patients at risk for invasive candidiasis. J Clin Microbiol. 2020;58:e01996-e2019.
Funding
Open access funding provided by University of Lausanne. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: MM, DPK, FL, MPO; methodology: PI, LS, AS, FK, FP, FF; validation: FF, DPK, FL; formal analysis: MM, DPK, FL, MPO; investigation: PI, LS, AS, ST, FK, SK, JLP, MV, FP, FF, MPO; resources: AS, FK, FP, DPK, FL; data curation, FL, MPO; writing—original draft preparation: FL, MPO; writing—review and editing: MM, PI, LS, AS, ST, FK, SK, JLP, MV, FP, FF, DPK; supervision, MM, DPK, FL; project administration: MPO.
Corresponding author
Ethics declarations
Conflict of interest
Frederic Lamoth declares research grants from Funginos, Novartis, Pfizer and Merck, and speaker honoraria from Gilead. All contracts were made with and fees paid to his institution (LUH). The remaining authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
The study adhered to the Declaration of Helsinki and was approved by the institutional ethics review boards; ethics committee of UGHP on 22.02.2022 (109.2022), ethics committee of UGHH on 27.06.2022 (643.2022), and ethics committee of Canton of Vaud on 07.10.2022 (2022-01532).
Consent to participate
Due to the retrospective nature of the study, all ethics committee waived the need of informed consent to participate. However, patients at LUH were excluded if they had previously refused to permit the use of their data for research purposes.
Consent to publish
Due to the retrospective nature of the study, all ethics committee waived the need of informed consent to participate. However, patients at LUH were excluded if they had previously refused to permit the use of their data for research purposes.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marangos, M., Ioannou, P., Senn, L. et al. Role of source control in critically ill candidemic patients: a multicenter retrospective study. Infection (2024). https://doi.org/10.1007/s15010-024-02222-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02222-z