Abstract
Background
Antimicrobial stewardship (AMS) programs are effective tools for improving antibiotic prescription quality. Their implementation requires the regular surveillance of antibiotic consumption at the patient and institutional level. Our study captured and analyzed antibiotic consumption density (ACD) for hospitalized pediatric patients.
Method
We collected antibacterial drug consumption data for 2020 from hospital pharmacies at 113 pediatric departments of acute care hospitals in Germany. ACD was calculated as defined daily dose (DDD, WHO/ATC Index 2019) per 100 patient days (pd). In addition, we analyzed the trends in antibiotic use during 2013–2020.
Results
In 2020, median ACD across all participating hospitals was 26.7 DDD/100 pd, (range: 10.1–79.2 DDD/100 pd). It was higher at university vs. non-university hospitals (38.6 vs. 25.2 DDD/100 pd, p < 0.0001). The highest use densities were seen on oncology wards and intensive care units at university hospitals (67.3 vs. 38.4 DDD/100 pd). During 2013–2020, overall ACD declined (− 10%) and cephalosporin prescriptions also decreased (− 36%). In 2020, cephalosporins nevertheless remained the most commonly dispensed class of antibiotics. Interhospital variability in cephalosporin/penicillin ratio was substantial. Antibiotics belonging to WHO AWaRe “Watch” and “Reserve” categories, including broad-spectrum penicillins (+ 31%), linezolid (+ 121%), and glycopeptides (+ 43%), increased over time.
Conclusion
Significant heterogeneity in ACD and prescription of different antibiotic classes as well as high prescription rates for cephalosporins and an increased use of reserve antibiotics indicate improvable antibiotic prescribing quality. AMS programs should urgently prioritize these issues to reduce antimicrobial resistance.
Similar content being viewed by others
Introduction
Increasingly, antimicrobial resistance has emerged as a global health problem—one linked to worse patient outcomes and increasing healthcare costs [1, 2]. Several studies have drawn connections between inappropriate antibiotic use and the development of antimicrobial resistance caused by selection pressure on both bacterial pathogens and on human flora microorganisms [1, 3, 4]. Antimicrobial resistance can develop after even a single dose of antibiotics and can persist for months afterward [5]. Furthermore, prolonged antimicrobial use is associated with toxicity. Even so, in up to 50% of cases, antimicrobials are prescribed inappropriately, either for treatment of infections or else as prophylaxis [6, 7]. For these reasons, it is urgent that antimicrobial use become managed more prudently. In 2015, a call for optimization of their use became part of the World Health Organization's (WHO) “Global action plan on antimicrobial resistance” [8]. Commonly labeled antimicrobial stewardship (AMS) programs, initiatives to improve antimicrobial prescribing have been developed. These have been proven effective also in pediatric settings in reducing antimicrobial prescription rates and related costs, as well lowering usage of reserve antibiotics and the antimicrobial resistance that accompanies it [9,10,11,12,13,14].
Besides reducing the overall antibiotic prescription rates by avoiding not-indicated or prolonged antibiotic treatments, the WHO emphasized on using antibiotics with a low risk for antimicrobial resistance whenever possible and classified antibiotics accordingly into the categories “Access”, “Watch”, and “Reserve” [15]. To reduce development of antimicrobial resistance, 60% of the total antibiotic consumption should consist of antibiotics belonging to access group antibiotics [16].
Crucial to every AMS program is the regular collection of antibiotic consumption data and the audit of antibiotic prescribing to identify potentially inappropriate prescribing and subsequently ameliorate it. Although the overall quantity of antibiotics prescribed to hospitalized children in Germany is small, the density of antibiotic consumption in this setting is high; 30–40% of children hospitalized in Germany receive at least one antibiotic for either therapy or prophylaxis [7, 17]. Unfortunately, a pool of regularly-collected, antibiotic consumption data is not available from pediatric hospitals in Germany. Therefore, the aim of our study was to analyze available antibiotic consumption data from the ADKA-if-DGI project (http://www.antiinfektiva-surveillance.de) in relation to hospital size and service type and to then record changes observed over the surveillance period 2013–2020.
Methods
Data were collected from German pediatric hospitals with acute care wards who were participants in the ADKA-if-DGI project. Quarterly hospital pharmacy reports on the quantity (number of units) of systemic antibiotics (oral or parenteral) dispensed to various wards or departments with unique cost centers were logged and converted into "Defined Daily Doses" (DDD, WHO/ATC Index 2019) [18]. Bed occupancy data were collected from the local hospital administration. Antibiotic consumption density (ACD) was expressed as DDD per 100 patient days, (pd, i.e., occupied bed days). Results were additionally calculated by employing “Recommended Daily Dose” (RDD) definitions of hospital-adapted doses for a variety of drugs that better reflect truly prescribed doses in hospitalized (adult) patients [19]. Details on data collection and analysis in the ADKA-if-DGI project have been described previously [20].
For the cross-sectional analysis, we calculated median and interquartile ranges (25% and 75% percentiles). Pooled means per year were used to describe longitudinal changes. Data were derived from general wards, intensive care wards (pediatric and/or neonatal ICUs), and hematology–oncology wards. Hospitals were divided into groups according to overall size (including non-pediatric beds): small hospitals (< 400 beds), medium-sized hospitals (400–800 beds), and large hospitals (> 800 beds). Among the large hospitals, university hospitals were evaluated separately. Data were compared using either the Wilcoxon rank-sum test for comparison of two groups, or else the Kruskal–Wallis test for comparison of more than two groups. All statistical tests were calculated with GraphPad Prism V.6 (GraphPad Software, La Jolla, CA, USA). These tests were two-tailed and considered significant if the p value was < 0.05. We also performed a panel analysis (several cross-sectional over several timepoints) using linear mixed effect models and by random for institutional effects in effects intercept and change over time [21]. Each hospital was coded by a random effect and time. Other co-variates such as hospital size and category as well as ward type were used to estimate antibiotic use over time and how this may be influenced by relevant factors. Comparisons between different antibiotic classes were done descriptively using generalized forest plots. This analysis used the Software R (Version 4.0.3. 2020-10-10 [22]) RStudio [23] and the packages lme4 [24] and metafor [25]. For assignment of antibiotic substances to displayed antibiotic classes and groups, see Supplementary Table 1. To describe consumption of cephalosporins, the consumption of 1st/2nd generation cephalosporins and 3rd/4th generation cephalosporins was added up. The consumption of penicillins was computed as sum of broad-spectrum penicillins, aminopenicillins/beta-lactamase inhibitors (BLI), and narrow-spectrum penicillins. For classification of antibiotics to WHO AWaRe groups, the WHO 2021 AWaRe classification was employed [15].
Results
Antibiotic consumption density in 2020
During 2020, a combination of 113 children's hospitals and pediatric divisions of acute care hospitals in Germany submitted a full year (four quarters) of antibiotic consumption data covering a total of 1,693,501 pd. Among the reporting hospitals were 36 hospitals with < 400 beds (32%), 36 with 400–800 beds (32%), and 41 (including 22 university hospitals) with > 800 beds (36%) (Table 1).
Median overall ACD at pediatric divisions of acute care hospitals was 26.7 DDD/100 pd, with an interquartile range of 20.1–36.3 (or median 20.7 RDD/100 pd, IQR 15–26.4) (Supplementary Table 2). Significantly higher ACD was found at university vs. non-university hospitals (p < 0.0001). At non-university hospitals, ACD increased in parallel with hospital size (Table 1). However, variability between individual hospitals, (which ranged from 10.1 to 79.2 DDD/100 pd), was high—a span corresponding to 7.8-fold difference among hospitals (Fig. 1). Interestingly, substantial variability in overall antibiotic use was also seen among hospitals of similar size (Fig. 1). For example, the difference in overall ACD among the 22 university hospitals was approximately 4.4-fold (79.2 DDD/100 pd, resp. 17.8 DDD/100 pd).
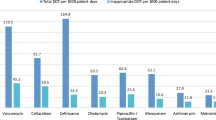
Antibiotic consumption density (in DDD/100 patient days) of specific antibiotic classes at 113 pediatric hospitals of different sizes and categories. A Hospitals with < 400 beds, B hospitals with 400–800 beds, C hospitals with > 800 beds. University hospitals are marked with a asterisk. Data are for the year 2020. BLI, beta-lactamase inhibitors
ACD was especially high on intensive care units (ICUs) and hematology/oncology wards of university hospitals (Table 2 and Supplementary Table 3). Here, the greatest disparity between university hospitals and non-university hospitals was observed. By contrast, on regular wards, ACD was similar for both university and non-university hospitals. Again however, increased ACD was observed to increase along with size of the non-university hospital on ICUs and regular wards.
Antibiotic classes
The five most common classes of antibiotics across all hospitals and wards in 2020 were (in DDD/100 pd): 1st/2nd generation cephalosporins, narrow-spectrum penicillins, 3rd/4th generation cephalosporins, aminopenicillins/BLI combinations, and macrolides/clindamycin (for RDD, see Supplementary Table 2). As with overall ACD, interhospital variability regarding prescription of different antibiotic classes was substantial (Fig. 1). This variability encompassed differences between cephalosporin and penicillin consumption (ratio ranging between 15:85 and 87:13), as well as that between piperacillin ± BLI and ampicillin/amoxicillin ± BLI, and that between 1st/2nd generation and 3rd/4th generation cephalosporins (Fig. 1).
As part of overall antibiotic consumption (in DDD), the proportion of broad-spectrum beta-lactams, (carbapenems, broad-spectrum penicillins and 3rd/4th generation cephalosporins), as well as their median ACD in DDD/100 pd, was significantly higher at university hospitals (31%; 11.2 DDD/100 pd) vs. non-university hospitals (21%; 4.6 DDD/100 pd (Fig. 2A and Supplementary Table 4). In comparing cephalosporin and penicillin consumption, however, the ratio remained similar between university (53:47) and non-university hospitals (57:43)—49:51 and 53:47 when calculated with RDD, respectively.
Use of antibiotic classes in patients in non-university and university hospitals (all wards) (A) and on regular hospital wards vs. ICU and oncologic wards of university hospitals (B) as a proportion of the median antibiotic consumption density (in % DDD) in the respective stratum. Data for the year 2020. BLI, beta-lactamase inhibitors
As expected, consumption of reserve and broad-spectrum antibiotics such as carbapenems, glycopeptides, and broad-spectrum penicillins was higher in ICU (especially at university hospitals) and oncology wards vs. in patients on regular wards. This is reflected in median DDD/100 and RDD/100, as well as in proportion of overall antibiotic use (Fig. 2B, Supplementary Table 4, and data not shown). For example, median usage of glycopeptides on ICUs and oncology wards at university hospitals was 30-fold and 49-fold higher than on regular wards at non-university hospitals (DDD/100 pd: 6.25 vs. 10.39 vs. 0.21, respectively).
AWaRe classification
According to the WHO AWaRe classification 2021, 63% of the antibiotics used during 2020 (in DDD) belong to the “Watch” and “Reserve” categories (Fig. 3A). While use of antibiotics from these categories varied extensively among individual hospitals (29 – 86%, Fig. 3B), higher consumption was observed in university vs. non-university hospitals (median 69.0% vs. 58.5%, p = 0.0013).
Longitudinal surveillance 2013–2020
The number of hospitals submitting data increased from 66 in 2013 to 113 in 2020, with 48 of the hospitals submitting data during every year of the surveillance period. From 2013 to 2020, overall ACD in DDD/100 pd decreased by 10% (p < 0.0001), a significant change (Table 3) observed at university and non-university hospitals (Fig. 5), as well as at hospitals of varying sizes (data not shown). The most notable decline was observed on hematology/oncology wards (p = 0.03)—the setting with the highest overall antibiotic consumption.
Consumption of penicillins increased significantly (+ 50.5%, p < 0.0001), whereas cephalosporins declined (− 35.7%, p < 0.0001, Fig. 4). Use of macrolides/clindamycin, tetracycline, and fluoroquinolones decreased over the 8-year period, while prescription of “reserve antibiotics” increased, especially at university hospitals (Fig. 5). These reserve antibiotics included broad-spectrum penicillins (piperacillin–tazobactam), carbapenems, glycopeptides, and linezolid (Table 3).
Overall antibiotic consumption density and consumption of single antibiotic classes as usage evolved during the course of the surveillance period (2013–2020) at university hospitals (A) and non-university hospitals (B). Data shown as a regression coefficient representing the change in ACD per year with 2.5%/97.5% confidence intervals. Statistics were calculated using a linear mixed model adjusted for hospital service type
Discussion
Our study collected and analyzed nationwide ACD at pediatric hospitals with acute care wards in Germany.
Overall ACD was 26.7 DDD/100 pd, with 25.3 DDD/100 pd for non-university and 38.6 DDD/100 pd for university hospitals in 2020. In comparison to findings from other European countries, this is on the lower range [26,27,28,29]. Variability of antibiotic use among the participating hospitals was striking, with a 7.8-fold difference in antibiotic use among the hospitals that had the lowest and the highest ACDs (Fig. 1). The higher ACD found in larger hospitals and in university hospitals vs. that seen in smaller hospitals and non-university hospitals may be explained by several factors: larger hospitals with > 800 beds mostly belong to tertiary care centers with specialized pediatric care units, and therefore treat many patients with underlying diseases (e.g., immunodeficiency, cystic fibrosis, post-kidney transplant, etc.). These patients are more prone to develop severe infections, to develop hospital-acquired infections, to be colonized by resistant microorganisms and to receive (broad-spectrum) antibiotics, factors that influence the ACD. Moreover, patients with severe infections/underlying conditions are often transferred to larger and university hospitals. In addition, hematology/oncology or bone marrow transplant wards, that were shown to have an extensively high antibiotic consumption, as well as NICUs caring for extremely low birth weight infants, are exclusively located in large (university) hospitals. Other institutional differences between small and large/non-university and non-university hospitals that may influence antibiotic prescription and consumption include existence of on-site infectious disease (ID) consulting services and/or AMS teams. Nevertheless, we also observed meaningful differences in ACD among participating university hospitals or between small hospitals with less than 400 beds, assuming comparable patient populations and treatment indications among these hospitals. This within-group variability suggests there to be either suboptimal adherence to national guidelines or else the existence of suboptimal local guidelines, ones that likely would benefit from the institution of a local AMS program [9, 14].
Several point or period prevalence surveys (PPS), that describe antibiotic prescribing practice at patient level, confirm our finding of a high variability in antibiotic consumption. In 2012, as part of the Antibiotic Resistance and Prescribing Project (ARPEC), a PPS from 23 German pediatric hospitals reported a similarly high variation in antibiotic prescribing practices [17, 30]. Here, antibiotic prevalence rates (APR) ranged from 6.5 to 49.5% (a 7.6-fold difference) among surveyed hospitals. The highest APR were recorded on oncology wards (65.0%), while the lowest were on general neonatal wards (7.3%). APR was higher at university hospitals (27.0%) as compared to non-university hospitals (16.7%). A British PPS study that examined the proportion of children who had been prescribed antibiotics, along with the DDD/100 pd per age group, also reported a wide variation in antibiotic use when comparing practices at the district general hospital and tertiary referral hospitals, as well as when examining the two types of hospitals separately [29].
Our study's finding that antibiotic use on hematology/oncology wards and ICUs was higher than on regular pediatric wards is in line with the results reported from several multicenter PPS from Europe and elsewhere [17, 29,30,31,32,33]. This finding may be explained by the high percentage of fever and severe infections, as well as the need for antimicrobial prophylaxis, in critically ill and/or vulnerable patient populations. Moreover, children who are on hematology/oncology/transplant wards or on ICUs are more likely to receive antibiotic combination therapies, a factor directly affecting the DDD/100 pd [17, 29]. However, several studies have shown that approximately half of antibiotic treatments on pediatric ICUs (PICUs) were inappropriate [32, 34, 35] and that significant reductions in antimicrobial use can be achieved on PICU and hematology/oncology wards (without increased adverse outcomes), when effective AMS programs, e.g., prospective audit with feedback or preauthorization systems, are implemented [36,37,38,39,40].
Assuming a similar case mix index and age structure in the participating hospitals over the surveillance period 2013–2020, our finding that antibiotic use among participating hospitals decreased over time is both noteworthy and relevant. Of particular interest is that the use of cephalosporins decreased by approximately 36%, whereas the usage of penicillins increased by 51%. According to the WHO AWaRe classification of antibiotics, penicillins are the preferred class of antibiotics for most pediatric indications and should replace 2nd, 3rd, or 4th generation cephalosporins whenever possible [41]. The use of cephalosporins is associated with the development of C. difficile infections (CDI) [42], as well as with the emergence of antibiotic-resistant microorganisms, including extended-spectrum beta-lactamase-producing Gram-negative bacteria [43, 44] and vancomycin-resistant enterococci [45]. The observed change in consumption density of penicillins and cephalosporins during the period 2013–2020 may be related to AMS initiatives successfully implemented in many German hospitals or the increasing availability of pediatric-specific antibiotic stewardship education programs—a positive development [10, 39, 46,47,48]. Unfortunately, however, our study found that in 2020, in both university and non-university hospitals, cephalosporins remained the most commonly used antibiotics across all pediatric departments (Table 3 and Supplementary Tables 2 and 4). This stayed true when RDD/100 pd was used as an alternative metric instead of DDD/100 pd, as DDD is based on a lower daily dose for cephalosporins, and therefore overestimates their use. In line with our conclusions, high consumption of cephalosporins (especially 3rd generation cephalosporins) has been identified via PPS in several other European countries as well. This emphasizes the need for a comprehensive, international effort to address antibiotic prescription quality [17, 49,50,51,52,53]. In some pediatric hospitals in Europe, including in Sweden, cephalosporin use has been successfully reduced over a 15-year period [54].
Despite overall decreases in antibiotic use (including that of cephalosporins), increased consumption of antibiotics belonging to the WHO “Watch” or “Reserve” categories—specifically, carbapenems, broad-spectrum penicillins, glycopeptides and linezolid — is concerning and needs further monitoring. According to a recent global PPS from the Global Antimicrobial Resistance, Prescribing, and Efficacy in Neonates and Children (GARPEC) and the Global PPS on Antimicrobial Consumption and Resistance (Global-PPS) networks, antibiotics belonging to the WHO “Watch” category in Europe and in Germany (five participating hospitals) were prescribed to pediatric patients at a rate of approximately 40% [51]. In our study, 61% of the dispensed antibiotics (in DDD) were antibiotics from the “Watch” category, despite substantial variations among hospitals. This misses the WHO target—a goal saying that at least 60% of total antibiotic consumption be from “Access” group antibiotics—by a long shot [16]. AMS activities as facility-specific treatment recommendations (including indication, choice of substance and duration of antibiotic treatment) or preauthorization systems for antibiotics belonging to “Watch” and “Reserve” categories might help to limit the extensive use of antibiotics from these categories [55, 56]. With 113 out of a total of 318 German pediatric hospitals participating during 2020 [57], our sample, which included both small and large hospitals, is of considerable size (36%). Because we do not have information on the specific populations served by each hospital participating in the study, we unfortunately are unable to determine the extent to which the selection of hospitals may be fully representative of all pediatric hospitals in Germany. Assessing antibiotic consumption as drug dispensing data in the form of DDD has its limitations, especially in pediatric populations as DDD usually represent average doses for adult patients in the community or hospital, rather than those effectively prescribed at the patient level, e.g., as days of therapy (DOTs) [58,59,60]. Therefore, no statements can be made regarding the quality of the single antibiotic prescription.
Moreover, although RDD/100 pd is closer to the actual dose of some antibiotics than DDD/100 pd in adult patients [61], calculations of both DDD and RDD are based on the average maintenance dose per day in adults and do not consider individual pediatric prescribing practices based upon age and/or body weight or body surface area (BSA). When dispensed by the pharmacy, either only a fraction of the antibiotic dose is given to the patient and the rest discarded, or else the dose might be divided among several pediatric patients. Correlation of DDD/RDD with actual consumption is dependent upon body weight variations in the studied population, upon the different drug vial sizes available at the hospital pharmacy, and upon the percentage of discarded drugs [62,63,64]. Therefore, DDD and RDD do not accurately reflect actual drug consumption on pediatric wards. Both overestimation and underestimation of drug use are of concern [33, 65, 66]. Furthermore, due to heterogeneity in patient age and body weight, as well as to variations in dosing schemes, comparability between wards and institutions might be impacted [59]. Metrics considered more appropriate for the capture of antibiotic consumption data in children include days of therapy, length of therapy or prescribed daily dose. Unfortunately, these data were not available to our study, as they generally require access to the individual patient, and collecting such data on a regular basis requires use of electronic health records to be cost-effective, which to date are not nationwide available [33, 47, 59, 66,67,68]. Nonetheless, given the variability of ACD even among university hospitals, which is less likely to be due only to differences in patient populations and/or regional antimicrobial resistance, we believe our findings remain valid despite these limitations. Moreover, DDD and RDD analyses offer the possibility of longitudinal surveillance of overall antibiotic consumption and/or particular antibiotic classes, assuming there to be a constant case mix [29, 59, 69]. Additional patient-level analyses in the form of point or period prevalence surveys (PPS) in the participating hospitals including information on the patient level (e.g., patient age, weight, underlying diseases) and antibiotic treatment (indication, length, dosage, documentation) could be used to confirm the data continuously assessed by analysis of pharmacy dispensing data and help us to get a better picture of ACD in pediatric care. This also would allow us to draw more specific conclusions regarding appropriateness of prescribed antibiotic treatments.
To conclude, despite the limitations of the DDD/100 method in a pediatric setting, our study indicates inappropriate use of antibiotics at several levels: (1) high variability in ACD between hospitals of similar size and service type; (2) the extensive use of antibiotics belonging to the WHO “Watch” and “Reserve” categories. Both topics should be addressed by antimicrobial stewardship activities. Longitudinal surveillance of the relatively easily accessible pharmacy dispensing data allows to assess the development of ACD and the effectiveness of AMS interventions on a national level. In addition, patient level analyses are needed to confirm and to complement the data regarding prescribing quality to specify existing AMS activities. The pipeline for new antibiotics has run dry. It is urgent that currently available antibiotics are used prudently [9, 12, 13].
Data availability statement
The data that support the findings of this study are available from the ADKA-if-DGI project upon reasonable request.
References
Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145. https://doi.org/10.3389/fpubh.2014.00145.
Aryee A, Price N. Antimicrobial stewardship—can we afford to do without it? Br J Clin Pharmacol. 2015;79:173–81. https://doi.org/10.1111/bcp.12417.
de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355:973–8. https://doi.org/10.1016/s0140-6736(00)90015-1.
van de Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14:1722–30. https://doi.org/10.3201/eid1411.070467.
Leach AJ, Shelby-James TM, Mayo M, Gratten M, Laming AC, Currie BJ, et al. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24:356–62. https://doi.org/10.1093/clinids/24.3.356.
Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2005:CD003543. https://doi.org/10.1002/14651858.CD003543.pub2.
Hufnagel M, Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, et al. High rates of prescribing antimicrobials for prophylaxis in children and neonates: results from the antibiotic resistance and prescribing in european children point prevalence survey. J Pediatr Infect Dis Soc. 2019;8:143–51. https://doi.org/10.1093/jpids/piy019.
Word Health Organisation. Global action plan on antimicrobial resistance. Geneve: 2015.
Donà D, Barbieri E, Daverio M, Lundin R, Giaquinto C, Zaoutis T, et al. Implementation and impact of pediatric antimicrobial stewardship programs: a systematic scoping review. Antimicrob Resist Infect Control. 2020;9:3. https://doi.org/10.1186/s13756-019-0659-3.
Kreitmeyr K, von Both U, Pecar A, Borde JP, Mikolajczyk R, Huebner J. Pediatric antibiotic stewardship: successful interventions to reduce broad-spectrum antibiotic use on general pediatric wards. Infection. 2017;45:493–504. https://doi.org/10.1007/s15010-017-1009-0.
Hersh AL, De Lurgio SA, Thurm C, Lee BR, Weissman SJ, Courter JD, et al. Antimicrobial stewardship programs in freestanding children’s hospitals. Pediatrics. 2015;135:33–9. https://doi.org/10.1542/peds.2014-2579.
Godbout EJ, Pakyz AL, Markley JD, Noda AJ, Stevens MP. Pediatric antimicrobial stewardship: state of the art. Curr Infect Dis Rep. 2018;20:39. https://doi.org/10.1007/s11908-018-0644-7.
Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J Pediatr Infect Dis Soc. 2015;4:e127-135. https://doi.org/10.1093/jpids/piu141.
Araujo da Silva AR, de Almeida Albernaz, Dias DC, Marques AF, Biscaia di Biase C, Murni IK, Dramowski A, et al. Role of antimicrobial stewardship programmes in children: a systematic review. J Hosp Infect. 2018;99:117–23. https://doi.org/10.1016/j.jhin.2017.08.003.
WHO. 2021 AWaRe classification 2021. https://www.who.int/publications/i/item/2021-aware-classification. Accessed 26 July 2023.
WHO. Adopt AWaRe 2019. https://adoptaware.org/. Accessed 25 July 2023.
Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, ARPEC project group. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71:1106–17. https://doi.org/10.1093/jac/dkv418.
WHOCC—ATC/DDD Index n.d. https://www.whocc.no/atc_ddd_index/. Accessed 25 June 2021.
ADKA-if-DGI Antiinfektiva-Surveillance, editor. Wirkstoffliste (DDD/RDD)—Stand 24.06.2019 n.d.
Kern WV, Fellhauer M, Hug M, Hoppe-Tichy T, Först G, Steib-Bauert M, et al. Antibiotika-Anwendung 2012/13 in 109 deutschen Akutkrankenhäusern. Dtsch Med Wochenschr. 2015;140:e237–46. https://doi.org/10.1055/s-0041-105938.
Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York, NY: Springer; 2000. https://doi.org/10.1007/b98969.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
RStudio Team. RStudio: Integrated Development Environment for R 2020.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67:1–48. https://doi.org/10.18637/jss.v067.i01.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36:1–48. https://doi.org/10.18637/jss.v036.i03.
Laine N, Hoppu K, Airaksinen M, Saxen H. Antimicrobial consumption in a tertiary children’s hospital in Finland (2003–2013). Eur J Hosp Pharm. 2016;23:266–71. https://doi.org/10.1136/ejhpharm-2015-000766.
Borzęcka B, Krasuski K, Kuchar EP. Antibiotic usage at a clinical paediatric hospital before and after the implementation of actions related to the hospital antibiotic policy. Eur J Hosp Pharm. 2021;28:207–11. https://doi.org/10.1136/ejhpharm-2019-001984.
Buccellato E, Melis M, Biagi C, Donati M, Motola D, Vaccheri A. Use of antibiotics in pediatrics: 8-years survey in Italian hospitals. PLoS ONE. 2015;10: e0139097. https://doi.org/10.1371/journal.pone.0139097.
Gharbi M, Doerholt K, Vergnano S, Bielicki JA, Paulus S, Menson E, et al. Using a simple point-prevalence survey to define appropriate antibiotic prescribing in hospitalised children across the UK. BMJ Open. 2016;6: e012675. https://doi.org/10.1136/bmjopen-2016-012675.
Hufnagel M, Madarova M, Rippberger B, Schuster K, Network P, Michel E. Antibiotic prescription pattern in 23 German pediatric hospitals: results from a point prevalence study. In: Poster at the 32 Annual Meeting of the European Society of Paediatric Infectious Diseases (ESPID), Abstract 0044, Dublin, Ireland; 2014.
Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H, et al. The Antibiotic Resistance and Prescribing in European Children Project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. 2013;32: e242. https://doi.org/10.1097/INF.0b013e318286c612.
Ceyhan M, Yildirim I, Ecevit C, Aydogan A, Ornek A, Salman N, et al. Inappropriate antimicrobial use in Turkish pediatric hospitals: a multicenter point prevalence survey. Int J Infect Dis. 2010;14:e55-61. https://doi.org/10.1016/j.ijid.2009.03.013.
D’Amore C, Ciofi Degli Atti ML, Zotti C, Prato R, Guareschi G, Spiazzi R, et al. Use of multiple metrics to assess antibiotic use in Italian children’s hospitals. Sci Rep. 2021;11:3543. https://doi.org/10.1038/s41598-021-83026-1.
Blinova E, Lau E, Bitnun A, Cox P, Schwartz S, Atenafu E, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med. 2013;14: e280. https://doi.org/10.1097/PCC.0b013e31828a846d.
Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med. 2000;26:959–66. https://doi.org/10.1007/s001340051288.
Aizawa Y, Suwa J, Higuchi H, Fukuoka K, Furuichi M, Kaneko T, et al. Antimicrobial stewardship program in a pediatric intensive care unit. J Pediatr Infect Dis Soc. 2018;7:e156–9. https://doi.org/10.1093/jpids/piy031.
Haque A, Hussain K, Ibrahim R, Abbas Q, Ahmed SA, Jurair H, et al. Impact of pharmacist-led antibiotic stewardship program in a PICU of low/middle-income country. BMJ Open Qual. 2018;7: e000180. https://doi.org/10.1136/bmjoq-2017-000180.
Stocker M, Ferrao E, Banya W, Cheong J, Macrae D, Furck A. Antibiotic surveillance on a paediatric intensive care unit: easy attainable strategy at low costs and resources. BMC Pediatr. 2012;12:196. https://doi.org/10.1186/1471-2431-12-196.
Renk H, Sarmisak E, Spott C, Kumpf M, Hofbeck M, Hölzl F. Antibiotic stewardship in the PICU: Impact of ward rounds led by paediatric infectious diseases specialists on antibiotic consumption. Sci Rep. 2020;10:8826. https://doi.org/10.1038/s41598-020-65671-0.
Wattier RL, Levy ER, Sabnis AJ, Dvorak CC, Auerbach AD. Reducing second gram-negative antibiotic therapy on pediatric oncology and hematopoietic stem cell transplantation services. Infect Control Hosp Epidemiol. 2017;38:1039–47. https://doi.org/10.1017/ice.2017.118.
WHO model list of essential medicines for children—7th list 2019. https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU201907. Accessed 26 Aug 2021.
Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1748–54. https://doi.org/10.1093/jac/dku046.
Gbaguidi-Haore H, Dumartin C, L’Hériteau F, Péfau M, Hocquet D, Rogues A-M, et al. Antibiotics involved in the occurrence of antibiotic-resistant bacteria: a nationwide multilevel study suggests differences within antibiotic classes. J Antimicrob Chemother. 2013;68:461–70. https://doi.org/10.1093/jac/dks406.
Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Fishman NO, Bilker WB, et al. Risk factors for increasing multidrug resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis. 2005;40:1317–24. https://doi.org/10.1086/429239.
McKinnell JA, Kunz DF, Chamot E, Patel M, Shirley RM, Moser SA, et al. Association between vancomycin-resistant Enterococci bacteremia and ceftriaxone usage. Infect Control Hosp Epidemiol. 2012;33:718–24. https://doi.org/10.1086/666331.
Metz J, Oehler P, Burggraf M, Burdach S, Behrends U, Rieber N. Improvement of guideline adherence after the implementation of an antibiotic stewardship program in a secondary care pediatric hospital. Front Pediatr. 2019;7:478. https://doi.org/10.3389/fped.2019.00478.
Huebner J, Hufnagel M, Liese J, Tenenbaum T, von Both U, Weichert S. S2k Leitlinie „Antibiotic Stewardship – Konzeption und Umsetzung in der stationären Kinder- und Jugendmedizin“; 2015.
DGPI: Deutsche Gesellschaft für Pädiatrische Infektiologie. https://dgpi.de/. Accessed 5 Sept 2023.
Tersigni C, Montagnani C, D’Argenio P, Duse M, Esposito S, Hsia Y, et al. Antibiotic prescriptions in Italian hospitalised children after serial point prevalence surveys (or pointless prevalence surveys): has anything actually changed over the years? Ital J Pediatr. 2019;45:127. https://doi.org/10.1186/s13052-019-0722-y.
Korinteli IG, Mchedlishvili I, Javakhadze M, Versporten A, Goossens H, Phagava H, et al. The global point prevalence survey (PPS) of antimicrobial use and antimicrobial resistance among hospitalized children in Georgia. Georgian Med News. 2019:72–5.
Hsia Y, Lee BR, Versporten A, Yang Y, Bielicki J, Jackson C, et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019;7:e861–71. https://doi.org/10.1016/S2214-109X(19)30071-3.
Krasniqi S, Versporten A, Jakupi A, Raka D, Daci A, Krasniqi V, et al. Antibiotic utilisation in adult and children patients in Kosovo hospitals. Eur J Hosp Pharm. 2019;26:146–51. https://doi.org/10.1136/ejhpharm-2017-001363.
Sviestina I, Usonis V, Gurksniene V, Burokiene S, Ivaskeviciene I, Mozgis D. Prescription of antibiotics in Riga and Vilnius tertiary children’s hospitals. Eur J Hosp Pharm. 2018;25:189–94. https://doi.org/10.1136/ejhpharm-2016-001124.
Luthander J, Bennet R, Nilsson A, Eriksson M. Antimicrobial use in a Swedish pediatric hospital: results from eight point-prevalence surveys over a 15-year period (2003–2017). Pediatr Infect Dis J. 2019;38:929–33. https://doi.org/10.1097/INF.0000000000002393.
Lee KR, Bagga B, Arnold SR. Reduction of Broad-Spectrum Antimicrobial Use in a Tertiary Children’s Hospital Post Antimicrobial Stewardship Program Guideline Implementation. Pediatr Crit Care Med. 2016;17:187–93. https://doi.org/10.1097/PCC.0000000000000615.
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–77. https://doi.org/10.1093/cid/ciw118.
Deutsche Gesellschaft für Kinder- und Jugendmedizin e.V. Kinderkliniken in Deutschland. Deutsche Gesellschaft für Kinder- und Jugendmedizin eV n.d. https://www.dgkj.de/veroeffentlichungen/kinderkliniken. Accessed 7 May 2022.
Fortin É, Fontela PS, Manges AR, Platt RW, Buckeridge DL, Quach C. Measuring antimicrobial use in hospitalized patients: a systematic review of available measures applicable to paediatrics. J Antimicrob Chemother. 2014;69:1447–56. https://doi.org/10.1093/jac/dku003.
Porta A, Hsia Y, Doerholt K, Spyridis N, Bielicki J, Menson E, et al. Comparing neonatal and paediatric antibiotic prescribing between hospitals: a new algorithm to help international benchmarking. J Antimicrob Chemother. 2012;67:1278–86. https://doi.org/10.1093/jac/dks021.
Muller A, Monnet DL, Talon D, Hénon T, Bertrand X. Discrepancies between prescribed daily doses and WHO defined daily doses of antibacterials at a university hospital. Br J Clin Pharmacol. 2006;61:585–91. https://doi.org/10.1111/j.1365-2125.2006.02605.x.
de With K, Bestehorn H, Steib-Bauert M, Kern WV. Comparison of defined versus recommended versus prescribed daily doses for measuring hospital antibiotic consumption. Infection. 2009;37:349–52. https://doi.org/10.1007/s15010-008-8138-4.
Baier J, Höpner J, Haase R, Diexer S, Stareprawo S, Mikolajczyk R, et al. Monitoring antibiotic consumption in pediatrics. How close to reality are days of therapy and recommended daily dose methods? Pediatr Infect Dis J. 2022;41:e126. https://doi.org/10.1097/INF.0000000000003446.
Liem TBY, Heerdink ER, Egberts ACG, Rademaker CMA. Quantifying antibiotic use in paediatrics: a proposal for neonatal DDDs. Eur J Clin Microbiol Infect Dis. 2010;29:1301–3. https://doi.org/10.1007/s10096-010-0990-3.
Mostaghim M, Snelling T, Bajorek B. Agreement between units of measure for paediatric antibiotic utilisation surveillance using hospital pharmacy supply data. Pharm Pract (Granada). 2019;17:1482–1482.
Amadeo B, Zarb P, Muller A, Drapier N, Vankerckhoven V, Rogues A-M, et al. European Surveillance of Antibiotic Consumption (ESAC) point prevalence survey 2008: paediatric antimicrobial prescribing in 32 hospitals of 21 European countries. J Antimicrob Chemother. 2010;65:2247–52. https://doi.org/10.1093/jac/dkq309.
Valcourt K, Norozian F, Lee H, Raszynski A, Torbati D, Totapally BR. Drug use density in critically ill children and newborns: analysis of various methodologies. Pediatr Crit Care Med. 2009;10:495–9. https://doi.org/10.1097/PCC.0b013e3181a3101e.
Dutey-Magni PF, Gill MJ, McNulty D, Sohal G, Hayward A, Shallcross L, et al. Feasibility study of hospital antimicrobial stewardship analytics using electronic health records. JAC Antimicrob Resist. 2021;3:dlab018. https://doi.org/10.1093/jacamr/dlab018.
Renggli L, Plüss-Suard C, Gasser M, Sonderegger B, Kronenberg A. Assessing the conversion of electronic medical record data into antibiotic stewardship indicators. J Antimicrob Chemother. 2023;78:2297–305. https://doi.org/10.1093/jac/dkad235.
Channon-Wells S, Kwok M, Booth J, Bamford A, Konstanty P, Hatcher J, et al. The use of continuous electronic prescribing data to infer trends in antimicrobial consumption and estimate the impact of stewardship interventions in hospitalized children. J Antimicrob Chemother. 2021;76:2464–71. https://doi.org/10.1093/jac/dkab187.
Acknowledgements
We thank all participating hospitals for their voluntary reporting of their pharmacy dispensing data. We thank Natalie Diffloth for her English language editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. The ADKA-if-DGI-project is financially supported by the German Society for Infectious Diseases (DGI), the Academy for Infection Medicine, the ADKA Bundesverband deutscher Krankenhausapotheker, and intramural funds from the Division of Infectious Diseases at the Freiburg University Medical Center. MF is supported as a fellow at the IMM-PACT-Program for Clinician Scientists, Department of Internal Medicine II, Medical Center—University of Freiburg and Faculty of Medicine, University of Freiburg. Her work is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—413517907.
Author information
Authors and Affiliations
Contributions
WVK, KW, and MFE conceived and designed the study; MS-B and MFR analyzed the data; MFR, MH, KW, and WVK wrote the paper; UM co-designed and supervised statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that might be construed as a potential conflict of interest.
Ethical approval
The conducted research is not related to either human or animals use.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Freudenhammer, M., Hufnagel, M., Steib-Bauert, M. et al. Antibiotic use in pediatric acute care hospitals: an analysis of antibiotic consumption data from Germany, 2013–2020. Infection (2023). https://doi.org/10.1007/s15010-023-02112-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-023-02112-w