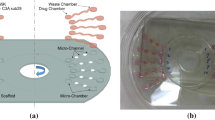
Abstract
During high-throughput drug screening, in vitro models are fabricated and the effects of therapeutics on the models evaluated in high throughput—for example, with automated liquid handling systems and microplate reader-based high-throughput screening (HTS) assays. The most frequently-used model systems for HTS, 2D models, do not adequately model the in vivo 3D microenvironment—an important aspect of which is the extracellular matrix—and therefore, 2D models may not be appropriate for drug screening. Instead, tissue-engineered 3D models with extracellular matrix-mimicking components are destined to become the preferred in vitro systems for HTS. However, for 3D models, such as 3D cell-laden hydrogels and scaffolds, cell sheets, and spheroids as well as 3D microfluidic and organ-on-a-chip systems, to replace 2D models in HTS, they must be compatible with high-throughput fabrication schemes and evaluation methods. In this review, we summarize HTS in 2D models and discuss recent studies that have successfully demonstrated HTS-compatible 3D models of high-impact diseases, such as cancers or cardiovascular diseases.






Similar content being viewed by others
References
Congressional Budget Office. Research and development in the pharmaceutical industry. 2021. https://www.cbo.gov/publication/57025.
Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012;134:82–106.
Joseph SJ, Malindisa ST, Ntwasa M. Two-dimensional (2D) and three-dimensional (3D) cell culturing in drug discovery. In: Cell culture. Mehanna RA, editor. IntechOpen. https://doi.org/10.5772/intechopen.81552
Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol. 2018;9:6.
Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EH, Koenderink GH. A guide to mechanobiology: where biology and physics meet. Biochim Biophys Acta. 2015;1853:3043–52.
Nam KH, Smith AS, Lone S, Kwon S, Kim DH. Biomimetic 3D tissue models for advanced high-throughput drug screening. J Lab Autom. 2015;20:201–15.
Yan X, Zhou L, Wu Z, Wang X, Chen X, Yang F, et al. High throughput scaffold-based 3D micro-tumor array for efficient drug screening and chemosensitivity testing. Biomaterials. 2019;198:167–79.
Somarathna M, Hwang PT, Millican RC, Alexander GC, Isayeva-Waldrop T, Sherwood JA, et al. Nitric oxide releasing nanomatrix gel treatment inhibits venous intimal hyperplasia and improves vascular remodeling in a rodent arteriovenous fistula. Biomaterials. 2022;280:121254.
Andukuri A, Minor WP, Kushwaha M, Anderson JM, Jun HW. Effect of endothelium mimicking self-assembled nanomatrices on cell adhesion and spreading of human endothelial cells and smooth muscle cells. Nanomedicine. 2010;6:289–97.
Ramaiahgari SC, den Braver MW, Herpers B, Terpstra V, Commandeur JN, van de Water B, et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol. 2014;88:1083–95.
Chen J, Zhang X, Millican R, Lynd T, Gangasani M, Malhotra S, et al. Recent progress in in vitro models for atherosclerosis studies. Front Cardiovasc Med. 2022;8:790529.
Attene-Ramos MS, Austin CP, Xia M. High throughput screening. In: Encyclopedia of toxicology. 3rd ed. Wexler P, editor. Academic Press; 2014. pp. 916–7.
Wildey MJ, Haunso A, Tudor M, Webb M, Connick JH. High-throughput screening. In: Annual reports in medicinal chemistry. Goodnow RA, editor. Academic Press; 2017. pp. 149–95.
Szymański P, Markowicz M, Olasik EM. Adaptation of high-throughput screening in drug discovery—toxicological screening tests. Int J Mol Sci. 2012;13:427–52.
Blay V, Tolani B, Ho SP, Arkin MR. High-throughput screening: today’s biochemical and cell-based approaches. Drug Discov Today. 2020;25:1807–21.
Etzion Y, Muslin AJ. The application of phenotypic high-throughput screening techniques to cardiovascular research. Trends Cardiovasc Med. 2009;19:207–12.
Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Cell viability assays. In: Assay guidance manual [Internet] Markossian S, Grossman A, Brimacombe K et al., editors. Bethesda (MD): Eli Lilly & company and the national center for advancing translational sciences; 2004.
Ghasemi M, Turnbull T, Sebastian S, Kempson I. The MTT assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 2021;22:12827.
Rampersad SN. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel). 2012;12:12347–60.
Invitrogen. Viability and Cytotoxicity Assay Reagents—Section 15.2. In: The molecular probes handbook. ThermoFisher Scientific. https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook/assays-for-cell-viability-proliferation-and-function/viability-and-cytotoxicity-assay-reagents.html. Accessed 28 Nov 2022.
Etzion Y, Hackett A, Proctor BM, Ren J, Nolan B, Ellenberger T, et al. An unbiased chemical biology screen identifies agents that modulate uptake of oxidized ldl by macrophages. Circ Res. 2009;105:148–57.
Invitrogen. Assays for cell enumeration, cell proliferation and cell cycle—Section 15.4. In: The molecular probes handbook. Thermofisher scientific. https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook/assays-for-cell-viability-proliferation-and-function/assays-for-cell-enumeration-cell-proliferation-and-cell-cycle.html. Accessed 28 Nov 2022.
Anantanawat K, Pitsch N, Fromont C, Janitz C. High-throughput Quant-iT picogreen assay using an automated liquid handling system. Biotechniques. 2019;66:290–4.
Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of picogreen reagent and development of a fluorescence-based solution assay for double-stranded dna quantitation. Anal Biochem. 1997;249:228–38.
Chiba T, Tsuchiya T, Mori R, Shimokawa I. Protein reporter bioassay systems for the phenotypic screening of candidate drugs: a mouse platform for anti-aging drug screening. Sensors (Basel). 2012;12:1648–56.
Promega. reporter genes and their applications. https://www.promega.com/resources/guides/cell-biology/bioluminescent-reporters/. Accessed 28 Nov 2022.
Dobbs M, Hughes D, Narahari J, Choi J, Los G, Webb B, et al. Performing dual-spectral luciferase assays using the BMG Labtech POLARstar Omega plate reader. Thermofisher scientific. 2013. https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/protein-biology-application-notes/simultaneous-dual-emission-detection-luciferase-reporter-assays.html. Accessed 28 Nov 2022.
Gomi K, Kajiyama N. Oxyluciferin, a luminescence product of firefly luciferase, is enzymatically regenerated into luciferin. J Biol Chem. 2001;276:36508–13.
Cutrona MB, Simpson JC. A high-throughput automated confocal microscopy platform for quantitative phenotyping of nanoparticle uptake and transport in spheroids. Small. 2019;15:1902033.
Warrick JW, Murphy WL, Beebe DJ. Screening the cellular microenvironment: a role for microfluidics. IEEE Rev Biomed Eng. 2008;1:75–93.
Yurdagul A Jr, Finney AC, Woolard MD, Orr AW. The arterial microenvironment: the where and why of atherosclerosis. Biochem J. 2016;473:1281–95.
Northcott JM, Dean IS, Mouw JK, Weaver VM. Feeling stress: the mechanics of cancer progression and aggression. Front Cell Dev Biol. 2018;6:17
Liu Q, Luo Q, Ju Y, Song G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol Med. 2020;17:282–92.
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131.
Seidman MA, Mitchell RN, Stone JR. Pathophysiology of atherosclerosis. In: Cellular and molecular pathobiology of cardiovascular disease. Willis MS, Homeister JW, Stone JR, editors. Academic Press; 2014. pp. 221–37.
Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, et al. Modeling physiological events in 2D vs 3D cell culture. Physiology (Bethesda). 2017;32:266–77.
Park D, Son K, Hwang Y, Ko J, Lee Y, Doh J, et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform). Front Immunol. 2019;10:1133.
Yu J, Lee S, Song J, Lee SR, Kim S, Choi H, et al. Perfusable micro-vascularized 3D tissue array for high-throughput vascular phenotypic screening. Nano Converg. 2022;9:16.
Chi CW, Lao YH, Rezwanuddin AHR, Benoy EC, Li C, Dereli-Korkut Z, et al. High-throughput tumor-on-a-chip platform to study tumor-stroma interactions and drug pharmacokinetics. Adv Healthc Mater. 2020;9e2000880.
Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro Nat Protoc. 2012;7:850–8.
Mosaad EO, Chambers KF, Futrega K, Clements JA, Doran MR. The microwell-mesh: a high-throughput 3D prostate cancer spheroid and drug-testing platform. Sci Rep. 2018;8:253.
Salerno A, Cesarelli G, Pedram P, Netti PA. Modular strategies to build cell-free and cell-laden scaffolds towards bioengineered tissues and organs. J Clin Med. 2019;8:1816.
Chiu LLY, Chu Z, Radisic M. Tissue engineering. In: Comprehensive nanoscience and technology. Andrews DL, Scholes GD, Wiederrecht GP, editors. Academic Press; 2011. pp. 175–211.
Xin X, Wu Y, Zhang R, Yang ST. A fluorescent 3D cell culture assay for high throughput screening of cancer drugs down-regulating survivin. J Biotechnol. 2019;289:80–7.
Martin HL, Adams M, Higgins J, Bond J, Morrison EE, Bell SM, et al. High-content, high-throughput screening for the identification of cytotoxic compounds based on cell morphology and cell proliferation markers. PLoS One. 2014;9:e88338.
Jaiswal PK, Goel A, Mittal RD. Survivin: a molecular biomarker in cancer. Indian J Med Res. 2015;141:389–97.
Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356:eaaf3627.
Sudhakar CK, Upadhyay N, Jain A, Verma A, Charyulu RN, Jain S. Hydrogels—promising candidates for tissue engineering. In: Nanotechnology applications for tissue engineering. Thomas S, Grohens Y, Ninan N, editors. William Andrew Publishing; 2015. pp. 77–94.
Lee SY, Hwang HJ, Ku B, Lee DW. Cell proliferation receptor-enhanced 3d high-throughput screening model for optimized drug efficacy evaluation in breast cancer cells. Anal Chem. 2022;94:11838–47.
Ma X, Dewan S, Liu J, Tang M, Miller KL, Yu C, et al. 3D printed micro-scale force gauge arrays to improve human cardiac tissue maturation and enable high throughput drug testing. Acta Biomater. 2019;95:319–27.
Lee J, Shin D, Roh JL. Development of an in vitro cell-sheet cancer model for chemotherapeutic screening. Theranostics. 2018;8:3964–73.
Han SB, Shin YJ, Hyon JY, Wee WR. Cytotoxicity of voriconazole on cultured human corneal endothelial cells. Antimicrob Agents Chemother. 2011;55:4519–23.
Chitty JL, Skhinas JN, Filipe EC, Wang S, Cupello CR, Grant RD, et al. The mini-organo: a rapid high-throughput 3d coculture organotypic assay for oncology screening and drug development. Cancer Rep (Hoboken). 2020;20:131.
Takezawa T, Mori Y, Yoshizato K. Cell culture on a thermo-responsive polymer surface. Biotechnology (N Y). 1990;8:854–6.
Metzger W, Sossong D, Bächle A, Pütz N, Wennemuth G, Pohlemann T, et al. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy. 2011;13:1000–12.
Nguyen LTH, Muktabar A, Tang J, Wong YS, Thaxton CS, Venkatraman SS, et al. The Potential of fluocinolone acetonide to mitigate inflammation and lipid accumulation in 2D and 3D foam cell cultures. Biomed Res Int. 2018. https://doi.org/10.1155/2018/3739251.eCollection.
Rakshit M, Darwitan A, Muktabar A, Das P, Nguyen LTH, Cao Y, et al. Anti-inflammatory potential of simvastatin loaded nanoliposomes in 2D and 3D foam cell models. Nanomedicine. 2021;37:102434.
Puls TJ, Tan X, Husain M, Whittington CF, Fishel ML, Harbin SLV. Development of a novel 3D tumor-tissue invasion model for high-throughput, high-content phenotypic drug screening. Sci Rep. 2018;8:13039.
Dykens JA, Will Y. Mitochondrial toxicity. In: Encyclopedia of toxicology (3rd edition), Wexler P, editor. Academic Press; 2014. pp. 349–53.
Invitrogen. Click Chemistry—Section 3.1. In: The molecular probes handbook. Thermofisher scientific. https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook/reagents-for-modifying-groups-other-than-thiols-or-amines/click-chemistry.html. Accessed 28 Nov 2022.
Hamelik RM, Krishan A. Click-iT™ assay with improved DNA distribution histograms. Cytom A. 2009. https://doi.org/10.1002/cyto.a.20780.
Leung CM, de Haan P, Bouchard KR, Kim G-A, Ko J, Rho HS, et al. A guide to the organ-on-a-chip. Nat Rev Methods Primers. 2022. https://doi.org/10.1038/s43586-022-00118-6.
Biotium. NucView Caspase-3 Substrates. https://biotium.com/technology/nucview-caspase-3-substrates/?keyword=%2Bcaspase%20inhibitors&creative=&msclkid=e7c42cf52a311c0c73b74f713737fd4d&utm_source=bing&utm_medium=cpc&utm_campaign=NucView&utm_term=%2Bcaspase%20inhibitors&utm_content=NucView. Accessed 28 Nov 2022.
Parrish J, Lim KS, Baer K, Hooper GJ, Woodfield TBF. A 96-well microplate bioreactor platform supporting individual dual perfusion and high-throughput assessment of simple or biofabricated 3D tissue models. Lab Chip. 2018;18:2757–75.
Hu X, Zhao S, Luo Z, Wang F, Zhu J, Chen L, et al. On-chip hydrogel arrays individually encapsulating acoustic formed multicellular aggregates for high throughput drug testing. Lab Chip. 2020;20:2228–36.
Hwang HH, You S, Ma X, Kwe L, Victorine G, Lawrence N, et al. High throughput direct 3D bioprinting in multiwell plates. Biofabrication. 2021. https://doi.org/10.1088/1758-5090/ab89c.
Yang C, Luo J, Polunas M, Bosnjak N, Chueng SD, Chadwick M, et al. 4D-printed transformable tube array for high-throughput 3d cell culture and histology. Adv Mater. 2020;32:e2004285.
Acknowledgment
Funding was provided by National Heart, Lung, and Blood Institute (Grant No. R01HL163802).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiment carried out for this article
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huskin, G., Chen, J., Davis, T. et al. Tissue-Engineered 3D In Vitro Disease Models for High-Throughput Drug Screening. Tissue Eng Regen Med 20, 523–538 (2023). https://doi.org/10.1007/s13770-023-00522-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00522-3