Abstract
Exacerbations or de novo autoimmune/autoinflammatory disease have been reported after COVID-19 vaccination. A young male presented with cutaneous IgA vasculitis with glomerular hematuria, diarrhea and pericarditis following his second COVID-19 mRNA vaccination. He also showed positivity for proteinase 3 anti-neutrophil cytoplasmic antibody (PR3-ANCA) and anti-cardiolipin antibody. Skin biopsy was compatible to IgA vasculitis. His purpura subsided and hematuria spontaneously disappeared. Treatment with anti-inflammatory medications and prednisolone resolved the pericarditis. He had a history of persistent diarrhea, and colonic biopsies showed possible ulcerative colitis without vasculitis. Kidney biopsy after prednisolone therapy revealed minor glomerular abnormalities without any immune reactants and did not show vasculitis. After prednisolone treatment, PR3-ANCA decreased in a medium degree despite of improvement of symptoms and inflammatory data, suggesting that his PR3-ANCA may be associated with ulcerative colitis. The cause of the transient glomerular hematuria was unclear, however, it might be caused by focal glomerular active lesions (glomerular vasculitis) due to vaccine-induced IgA vasculitis with nephritis. This case highlights that COVID-19 mRNA vaccination can activate multiple autoimmune/autoinflammatory systems. The conditions might help us better understand the mutual mechanisms of the relevant disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vaccines have been shown to trigger an immune response that leads to a broad spectrum of autoimmune diseases [1]. Watad et al. reported the first large series description of new-onset or flare of immune-mediated diseases such as pericarditis, IgA vasculitis (IgAV), myasthenia gravis, multiple sclerosis and arthritis temporally associated with COVID-19 mRNA vaccination [2]. Associations between COVID-19 mRNA vaccines and new-onset or recurrent glomerular diseases have also been reported [3].
We describe a young male who developed cutaneous manifestations of IgAV with glomerular hematuria, diarrhea and pericarditis after receiving his second dose of COVID-19 mRNA vaccine. He also showed positivity for proteinase-3 anti-neutrophil cytoplasmic antibody (PR3-ANCA) and anti-cardiolipin antibody. The features with activation of multiple autoimmune/autoinflammatory systems in our case are extremely rare. The possible clinicopathological interpretation is discussed.
Case report
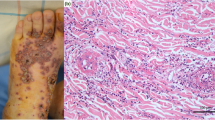
An 18-year-old male presented to our emergency room with a fever of 39.0 °C and walking difficulty due to the tenderness of the edematous right lower limb. Palpable purpura of up to 5 mm size were scattered on both feet and one 3 mm size blood blister was observed. He received his second dose of the Pfizer-BioNTech mRNA COVID-19 vaccine 6 days prior and had a fever and purpura/erythema in both lower legs 1 day after vaccination. His clinical picture was shown in Fig. 1. The patient was treated with topical clobetasol propionate ointment and oral levocetirizine hydrochloride. However, the eruption expanded to both lower limbs. Laboratory data observed at the dermatology unit 8 days after vaccination are shown in Table 1. C-reactive protein (CRP) was positive. Trace protein and microscopic hematuria with dysmorphic red blood cells (RBCs) in urine were found. Skin biopsy revealed a neutrophilic infiltrate with leukocytoclasia around the blood vessels with IgA deposition (Fig. 2A-C), which was compatible with IgAV. However, his PR-3 ANCA and anti-cardiolipin IgG titers were elevated. Computed tomography of the chest and paranasal sinus did not show any lesions suggesting granulomatosis with polyangiitis or pulmonary hemorrhage. Kidney biopsy was planned for evaluation of nephritic syndrome. Diarrhea began 10 days after vaccination. 14 days after vaccine shot, he came to our emergency room complaining of chest pain upon deep breathing. His electrocardiogram (ECG) showed normal sinus rhythm with ST-segment elevation in leads I, II, aVF and V2-6, suggesting acute pericarditis (Fig. 3). He was admitted to our hospital.
The findings of skin biopsy. A Inflammatory cell infiltration in the upper dermis (arrow). Hematoxylin–eosin staining. Original magnification × 100. Bar = 200 μm. B A neutrophilic infiltrate with leukocytoclasia (asterisks) and eosinophils (arrowheads) are found around the blood vessel consists of endothelial cells with nuclear swelling (arrows). Hematoxylin–eosin staining. Original magnification × 400. Bar = 20 μm. C Direct immunofluorescence shows IgA deposits within upper dermal blood vessels
He had a history of childhood asthma and atopic dermatitis and sometimes had persistent and repetitive diarrhea and abdominal pain over the last 3 years. Ramosetron hydrochloride was prescribed for a diagnosis of irritable bowel syndrome without colonoscopic examination. He did not have a history of COVID-19 infection. Physical examination showed alert consciousness, body temperature of 37.3 °C, blood pressure of 95/43 mmHg and heart rate of 111/min. All the eruptions completely healed 15 days after vaccine shot. His other physical examinations were unremarkable. The polymerase chain reaction test for COVID-19 was negative. His troponin T level was normal. Chest X-ray showed no acute cardiopulmonary changes. The echocardiogram showed a normal ejection fraction of 61% and no regional wall motion changes or pericardial effusion. He was tentatively diagnosed with post-COVID-19 vaccination pericarditis and treated with loxoprofen sodium hydrate and colchicine. His chest pain did not completely disappear, so oral prednisolone 30 mg/day was started 3 days after the appearance of chest pain. On the same day his glomerular hematuria was confirmed to be normalized. His chest pain soon resolved after corticosteroid administration. Serology for common viral causes of pericarditis, such as echovirus, influenza virus types A and B, coxsackievirus groups A and B, and human immunodeficiency virus, was negative.
He also complained of slight abdominal pain and diarrhea, which resolved 3 days after corticosteroid therapy. Esophagogastroduodenoscopy showed benign erosion in the stomach without findings of vasculitis in the biopsy specimen. Colonoscopy revealed nonspecific colitis in the ascending colon, transverse colon and sigmoid colon, suggesting inflammatory bowel disease or infectious colitis. Colonic biopsy showed mildly to severely inflammatory cell infiltration with lymphocyte and eosinophil in the lamina propria and mild crypt structural alterations in the ascending colon and transverse colon (Fig. 4A). In the sigmoid colon, widespread disappearance of the epithelium and erosion replaced with inflammatory granulation tissue in association with severely inflammatory infiltrated cells with lymphocyte and eosinophil were seen (Fig. 4B, C). There was no vasculitis, but vascular proliferation and endothelial swelling were found within inflammatory granulation tissue (Fig. 4D). Immunohistochemistry for cytomegalovirus was negative. These acute-on-chronic inflammatory changes did not contradict active ulcerative colitis (UC).
A colonic biopsy. A Mildly to severely inflammatory infiltration cell with lymphocyte and eosinophil in the lamina propria and mild crypt structural alterations in the transverse colon is found. Hematoxylin–eosin staining. Original magnification × 100. Bar = 100 μm. B Widely disappeared epithelium with erosion and inflammatory granulation tissue associated with many inflammatory infiltrated cells are found in the sigmoid colon. Hematoxylin–eosin staining. Original magnification × 100. Bar = 100 μm. C Eosinophils are found in the inflammatory granulation tissue (arrows). Hematoxylin–eosin staining. Original magnification × 200. Bar = 20 μm. D The area of the square in Fig. 3B shows vascular proliferation and endothelial swelling without vasculitis within inflammatory granulation tissue (arrows). Hematoxylin–eosin staining. Original magnification × 200. Bar = 20 μm
Although his glomerular hematuria had spontaneously disappeared before corticosteroid therapy, kidney biopsy was performed 9 days after corticosteroid administration to evaluate the presence of IgAV nephritis or ANCA-associated glomerulonephritis. Kidney biopsy revealed minor glomerular abnormalities of 58 glomeruli and no tubulointerstitial lesions. Two glomeruli included 5 polymorphonuclear leukocytes (Fig. 5A) and 8 glomeruli had 1–3 polymorphonuclear leukocytes. Erythrocytes in a group were found in some tubular lumens (Fig. 5B). There were no depositions of immune reactants on immunofluorescence and no electron-dense deposits on electron microscopy. The glomerular basement membrane thickness was within the normal range between 250 and 350 nm on electron microscopy.
Kidney biopsy findings. A A light micrograph shows a glomerulus with a few polymorphonuclear leukocytes (arrows) in the glomerular capillary walls. Original magnification × 400. Periodic acid-Schiff. Bar = 100 μm. B There are erythrocytes in a group in some proximal tubular lumens (asterisks). Original magnification × 200. Hematoxylin–eosin/Periodic acid-methenamine silver staining. Bar = 50 μm
He was discharged with a plan of tapering the prednisolone with careful observation of any symptoms or laboratory findings related to vasculitis. Although his CRP level and urinalysis had been normal and his ECG had normalized, the PR3-ANCA titer remained high, suggesting that PR3-ANCA may not be associated with ANCA-associated vasculitis. He again complained of diarrhea and abdominal pain when prednisolone was tapered to 12.5 mg/day at a follow-up 4.5 months after corticosteroid therapy. The repeated colonoscopy with colonic biopsy showed nonspecific colitis again. However, he showed bloody stool afterwards. Therefore, he was diagnosed as possible UC and 5-aminosalicylic acid was started. The PR3-ANCA titer had been maintained with about 200 U/mL, but anti-cardiolipin antibody titer gradually normalized.
Discussion
A direct causal relationship between COVID-19 vaccination and autoimmune/autoinflammatory disease has yet to be firmly established. Vojdani et al. [4] demonstrated that anti-severe acute respiratory syndrome coronavirus (SARS-CoV-2) antibodies reacted with 28 tissue antigens, representing diverse tissue groups that included barrier proteins, gastrointestinal, thyroid and neural tissues, and more, suggesting the potential risk for autoimmunity with COVID-19 vaccination.
So far, 1 case of uncertainty of pre-existing of IgAV, 3 cases of reactivation and 8 cases of new-onset of IgAV following COVID-19 vaccination have been reported in detail [5,6,7,8,9,10,11,12,13,14,15,16]. Nine cases received mRNA vaccine. Interestingly, a link between the increase in anti-SARS-CoV-2 spike IgA and the reactivation of pre-existing IgAV after COVID-19 vaccination has been suggested [8]. Eight patients showed symptoms of IgAV after the first dose, and 4 showed symptoms approximately 7–20 days after the second dose. Eight cases were associated with renal involvement, and among them only one case showed gross hematuria and others microscopic hematuria. Most cases showed self-remission or relatively early resolution after corticosteroid treatment. In our case, the purpura and glomerular hematuria improved spontaneously, within approximately 2 weeks after the onset like many reported cases. Kidney biopsy after corticosteroid treatment did not show any vasculitic changes. However, the focal existence of intraglomerular polymorphonuclear leukocytes and intratubular erythrocytes might indicate residual glomerular inflammation. It is unlikely that the kidney findings resulted from ANCA-associated glomerulonephritis because some glomerular injuries should have remained in the early phase of corticosteroid treatment.
IgAV nephritis and IgA nephropathy (IgAN) have been shown to share the mesangial deposition of galactose-deficient IgA1 [17], suggesting similar disease mechanisms of them. It was reported that the sequential events involving nephritogenic IgA from IgAN-prone mice showed that injected IgA had strong affinity to mesangial, subepithelial and subendothelial lesions with acute glomerular cellular activation in nude mice, suggesting all glomerular cellular elements are plausible target for injuries which explain the mechanisms leading to hematuria [18]. The deposits disappeared 24-h after single nephritogenic IgA injection, indicating that glomerular IgA deposition in IgAN may be expressed under the balance between deposition and clearance [18]. We speculated that IgA deposits could be removed spontaneously if the production period of nephritogenic IgA is transient, and glomerular inflammation can almost subside with corticosteroid treatment before kidney biopsy. Interestingly, it was suggested that rupture of focal glomerular basement membranes caused by intracapillary leukocyte influx (glomerular vasculitis) is the main progressive factor in the early stage of IgAN other than the mesangial IgA deposition [19]. This might be a mechanism of glomerular hematuria in our patient. Therefore, it is conceivable that the glomerular hematuria in our case was derived from transient mechanisms causing IgAV nephritis following COVID-19 vaccination. Detection and investigation of patients with new-onset IgAV nephritis/IgAN after COVID-19 vaccination are a unique opportunity to understand the trigger mechanisms.
The most common presentation after COVID-19 vaccine myocarditis or pericarditis is chest pain, followed by fever, and rarely headache, cough, and dyspnea [20]. Symptoms appear approximately 2–3 days after vaccination, most often after the second dose, and are associated with elevated troponin and CRP and an ECG with ST elevations in most reports [20]. Our patient did not have significant signs of myocardial injury, arrhythmia, or hemodynamic instability, refuting myocarditis. Most reported cases require hospitalization for up to 4 days but are still considered mild [20]. Our case also had a favorable clinical course.
We thought that our patient had possible UC before the first dose of COVID-19 vaccine based on his clinical manifestations and pathology [21]. His UC might be exacerbated by vaccine shot. So far, only one case with UC flare-ups following COVID-19 mRNA vaccination in the setting of long-term remission history was reported [22]. It has been reported that PR3-ANCAs are associated with UC in adults [23], and PR3-ANCA by chemiluminescence immunoassay was reported to be highly specific for distinguishing between UC and Crohn's disease [24]. Interestingly, it was also shown in adults that PR3-ANCA reactivity decreases with UC duration, being 48.5% with 0–2 years and 16.7% with 17–20 years [23]. The persistent positivity of PR3-ANCA without vasculitic features after corticosteroid treatment in our patient may have been associated with UC.
A significant number of patients with inflammatory bowel disease are positive for antiphospholipid antibodies [25] or anti-cardiolipin antibodies [26] without thrombotic events. On the other hand, a few cases of thrombocytopenia and thrombotic events resembling antiphospholipid syndrome have been reported to develop following COVID-19 vaccination. In these cases, the pathogenesis is thought to be related to acquired thrombosis [27]. Though his fibrinogen and D-dimer were transiently elevated before admission, our patient did not show acquired thrombosis on ultrasound screening. Therefore, his anti-cardiolipin might have been also related to pre-existing UC.
The present case highlights that COVID-19 vaccination can activate multiple autoimmune/autoinflammatory systems because it is difficult to understand that these observations are merely coincidental. Pre-existing UC as autoinflammatory disease might have made the patient prone to develop the conditions he did following COVID-19 vaccination. It is likely that similar triggering mechanisms may be responsible for causing an autoimmune-mediated reaction in the situations. Toll-like receptors are thought to play a role in the pathogenesis of IgAV/IgAN, pericarditis/myocarditis and UC [28,29,30]. After uptake of double-stranded RNA byproducts in COVID-19 mRNA vaccine it may stimulate Toll-like receptors, resulting in a cascade of intracellular processes activating an innate immunity-driven cascade of humoral- and cell-mediated immunity [29]. It is hoped that an accumulation of analyses of the activation of multiple autoimmune/autoinflammatory systems by vaccination will help us better understand the mechanisms of the relevant disorders.
References
Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360:1981–8.
Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, Brodavka M, Cohen Y, Abu-Much A, Abu Elhija M, Bridgewood C, Langevitz P, McLorinan J, Bragazzi NL, Marzo-Ortega H, Lidar M, Calabrese C, Calabrese L, Vital E, Shoenfeld Y, Amital H, McGonagle D. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel). 2021;9:435.
Chan ATP, Tang SCW. De novo and relapsing glomerulonephritis after COVID-19 vaccination: how much do we know? Nephrology (Carlton). 2022;27:5–6.
Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11: 617089.
Hines AM, Murphy N, Mullin C, Barillas J, Barrientos JC. Henoch-Schönlein purpura presenting post-COVID-19 vaccination. Vaccine. 2021;39:4571–2.
Naitlho A, Lahlou W, Bourial A, Rais H, Ismaili N, Abousahfa I, Belyamani L. A rare case of Henoch-Schönlein purpura following a COVID-19 vaccine-case report. SN Compr Clin Med. 2021;8:1–4.
Sirufo MM, Raggiunti M, Magnanimi LM, Ginaldi L, De Martinis M. Henoch Schönlein purpura following the first dose of COVID-19 viral vector vaccine: a case report. Vaccines (Basel). 2021;9:1078.
Obeid M, Fenwick C, Pantaleo G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. 2021;3: e617.
Kondo M, Yamanaka K. Possible HSP reactivation post-COVID-19 vaccination and booster. Clin Case Rep. 2021;9: e05032.
Mohamed MMB, Wickman TJ, Fogo AB, Velez JCQ. De novo immunoglobulin a vasculitis following exposure to SARS-CoV-2 immunization. Ochsner J. 2021;21:395–401.
Badier L, Toledano A, Porel T, Dumond S, Jouglen J, Sailler L, Bagheri H, Moulis G, Lafaurie M. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV-19. Autoimmun Rev. 2021;20: 102951.
Maye JA, Chong HP, Rajagopal V, Petchey W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. 2021;14: e247188.
Grossman ME, Appel G, Little AJ, Ko CJ. Post-COVID-19 vaccination IgA vasculitis in an adult. J Cutan Pathol. 2021. https://doi.org/10.1111/cup.14168.
Iwata H, Kamiya K, Kado S, Nakaya T, Kawata H, Komine M, Ohtsuki M. Case of immunoglobulin A vasculitis following coronavirus disease 2019 vaccination. Dermatol. 2021;48:e598–9.
Nakatani S, Mori K, Morioka F, Hirata C, Tsuda A, Uedono H, Ishimura E, Tsuruta D, Emoto M. New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: case report. CEN Case Rep. 2022;25:1–5.
Hashizume H, Ajima S, Ishikawa Y. Immunoglobulin A vasculitis post-severe acute respiratory syndrome coronavirus 2 vaccination and review of reported cases. J Dermatol. 2022;49:560–3.
Suzuki H, Yasutake J, Makita Y, Tanbo Y, Yamasaki K, Sofue T, Kano T, Suzuki Y. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93:700–5.
Yamaji K, Suzuki Y, Suzuki H, Satake K, Horikoshi S, Novak J, Tomino Y. The kinetics of glomerular deposition of nephritogenic IgA. PLoS ONE. 2014;9: e113005.
Hotta O, Ieiri N, Nagai M, Tanaka A, Harabuchi Y. Role of palatine tonsil and epipharyngeal lymphoid tissue in the development of glomerular active lesions (glomerular vasculitis) in immunoglobulin A nephropathy. Int J Mol Sci. 2022;23:727.
Hana D, Patel K, Roman S, Gattas B, Sofka S. Clinical cardiovascular adverse events reported post-COVID-19 vaccination: are they a real risk? Curr Probl Cardiol. 2022;47: 101077.
Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, Kato J, Kobayashi K, Kobayashi K, Koganei K, Kunisaki R, Motoya S, Nagahori M, Nakase H, Omata F, Saruta M, Watanabe T, Tanaka T, Kanai T, Noguchi Y, Takahashi KI, Watanabe K, Hibi T, Suzuki Y, Watanabe M, Sugano K, Shimosegawa T. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–53.
Wang Y, Hsieh TC, Lee GY, Gorelick S, Maslak D. Ulcerative colitis flare-ups following mRNA COVID-19 vaccination. Am J Gastroenterology. 2021;116(SUPPL):S1029.
Mahler M, Bogdanos DP, Pavlidis P, Fritzler MJ, Csernok E, Dam Oiseaux J, Bentow C, Shums Z, Forbes A, Norman GL. PR3-ANCA: a promising biomarker for ulcerative colitis with extensive disease. Clin Chim Acta. 2013;424:267–73.
Horn MP, Peter AM, Righini Grunder F, Leichtle AB, Spalinger J, Schibli S, Sokollik C. PR3-ANCA and panel diagnostics in pediatric inflammatory bowel disease to distinguish ulcerative colitis from Crohn’s disease. PLoS ONE. 2018;13: e0208974.
Chiarantini E, Valanzano R, Liotta AA, Cellai AP, Fedi S, Ilari I, Prisco D, Tonelli F, Abbate R. Hemostatic abnormalities in inflammatory bowel disease. Thromb Res. 2019;82:137–46.
Koutroubakis IE, Petinaki E, Anagnostopoulou E, Kritikos H, Mouzas IA, Kouroumalis EA, Manousos ON. Anti-cardiolipin and anti-beta2-glycoprotein I antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1998;43:2507–12.
Talotta R, Robertson ES. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: the straw that breaks the camel’s back? Cytokine Growth Factor Rev. 2021;60:52–60.
Donadio ME, Loiacono E, Peruzzi L, Amore A, Camilla R, Chiale F, Vergano L, Boido A, Conrieri M, Bianciotto M, Bosetti FM, Coppo R. Toll-like receptors, immunoproteasome and regulatory T cells in children with Henoch-Schönlein purpura and primary IgA nephropathy. Pediatr Nephrol. 2014;29:1545–51.
Milano G, Gal J, Creisson A, Chamorey E. Myocarditis and COVID-19 mRNA vaccines: a mechanistic hypothesis involving dsRNA. Future Virol. 2021. https://doi.org/10.2217/fvl-2021-0280.
Dai W, Long L, Wang X, Li S, Xu H. Phytochemicals targeting Toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin Med. 2022;17:53.
Acknowledgements
We would like to thank Ms. Hiromi Yamaguchi for her technical assistance.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal participants
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ito, C., Odajima, K., Niimura, Y. et al. IgA vasculitis with transient glomerular hematuria, diarrhea, and pericarditis following COVID-19 mRNA vaccination in a young patient with possible pre-existing ulcerative colitis. CEN Case Rep 12, 84–90 (2023). https://doi.org/10.1007/s13730-022-00727-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-022-00727-w