Abstract
Purpose of Review
This narrative review aimed to explore the functions of betaine and discuss its role in patients with chronic kidney disease (CKD).
Recent Findings
Some studies on CKD animal models have shown the benefits of betaine supplementation, including decreased kidney damage, antioxidant recovery status, and decreased inflammation.
Summary
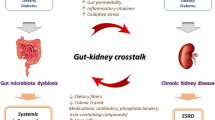
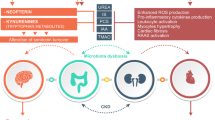
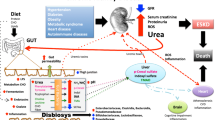
Betaine (N-trimethylglycine) is an N-trimethylated amino acid with an essential regulatory osmotic function. Moreover, it is a methyl donor and has anti-inflammatory and antioxidant properties. Additionally, betaine has positive effects on intestinal health by regulating the osmolality and gut microbiota. Due to these crucial functions, betaine has been studied in several diseases, including CKD, in which betaine plasma levels decline with the progression of the disease. Low betaine levels are linked to increased kidney damage, inflammation, oxidative stress, and intestinal dysbiosis. Furthermore, betaine is considered an essential metabolite for identifying CKD stages.



Similar content being viewed by others
References
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2014;506(7489):516.
Miller AC. Erwartungsbildung ökonomischer Akteure. Erwartungsbildung ökonomischer Akteure. 2003.
Craig SAS. Betaine in human nutrition. Am J Clin Nutr 2004;539–49.
Kim SK, Kim YC. Effects of betaine supplementation on hepatic metabolism of sulfur-containing amino acids in mice. J Hepatol. 2005;42:907–13.
Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC Natl Acad Press. Washington, D.C.: National Academies Press. 1998.
Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43:732–44.
Pourmehdi A, Sakhaei Z, Alirezaei M, Dezfoulian O. Betaine effects against asthma-induced oxidative stress in the liver and kidney of mice. Mol Biol Rep. 2020;47:5729–35.
Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34:3–15.
Dibaba DT, Johnson KC, Kucharska-Newton AM, Meyer K, Zeisel SH, Bidulescu A. The Association of Dietary Choline and Betaine With the Risk of Type 2 Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2020;43:2840–6.
Day CR, Kempson SA. Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta - Gen Subj. 2016;1098–106.
Moreira L de SG, Fanton S, Cardozo L, Borges NA, Combet E, Shiels PG, et al. Pink pressure: beetroot ( Beta vulgaris rubra) as a possible novel medical therapy for chronic kidney disease. Nutr Rev. 2021;nuab074.
Nelson AJ, Raggi P, Wolf M, Gold AM, Chertow GM, Roe MT. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic to Transl Sci Elsevier. 2020;398–412.
Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018.
Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL, Arthur JM. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol. Am J Physiol Renal Physiol; 2019;316:F1211–7.
Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci Portland Press Ltd. 2018;509–22.
Mafra D, Esgalhado M, Borges NA, Cardozo LFMF, Stockler-Pinto MB, Craven H, et al. Methyl donor nutrients in chronic kidney disease: impact on the epigenetic landscape. J Nutr J Nutr. 2019;149:372–80.
Gil RB, Ortiz A, Sanchez-Niño MD, Markoska K, Schepers E, Vanholder R, et al. Increased urinary osmolyte excretion indicates chronic kidney disease severity and progression rate. Nephrol Dial Transplant Oxford Academic. 2018;33:2156–64.
Velenosi TJ, Thomson BKA, Tonial NC, RaoPeters AAE, Mio MA, Lajoie GA, et al. Untargeted metabolomics reveals N, N, N-trimethyl-L-alanyl-L-proline betaine (TMAP) as a novel biomarker of kidney function. Sci Rep. Nature Publishing Group. 2019;9:1–13.
Guo F, Dai Q, Zeng X, Liu Y, Tan Z, Zhang H, et al. Renal function is associated with plasma trimethylamine-N-oxide, choline, L-carnitine and betaine: a pilot study. Int Urol Nephrol. 2021;53:539–51.
Alirezaei M, Jelodar G, Niknam P, Ghayemi Z, Nazifi S. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J Physiol Biochem. 2011;67:605–12.
Hagar H, El Medany AE, Salam R, El Medany GE, Nayal OA. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol. 2015;67:133–41.
Ghartavol MM, Gholizadeh-Ghaleh Aziz S, Babaei G, Hossein Farjah G, Hassan Khadem Ansari M. The protective impact of betaine on the tissue structure and renal function in isoproterenol-induced myocardial infarction in rat. Mol Genet Genomic Med. 2019;7:1–8.
Scheibler C. Ueber das Betain, eine im Safte der Zuckerrüben (Beta vulgaris) vorkommende Pflanzenbase. Berichte der Dtsch Chem Gesellschaft. John Wiley & Sons, Ltd. 1869;2:292–5.
Filipčev B, Kojić J, Krulj J, Bodroža-Solarov M, Ilić N. Betaine in cereal grains and grain-based products. Foods. 2018;7.
Barak AJ, Tuma DJ. Betaine, metabolic by-product or vital methylating agent? Life Sci Pergamon. 1983;32:771–4.
De Zwart FJ, Slow S, Payne RJ, Lever M, George PM, Gerrard JA, et al. Glycine betaine and glycine betaine analogues in common foods. Food Chem Elsevier. 2003;83:197–204.
Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–7.
Slow S, Donaggio M, Cressey PJ, Lever M, George PM, Chambers ST. The betaine content of New Zealand foods and estimated intake in the New Zealand diet ARTICLE IN PRESS. J Food Compos Anal. 2005;18:473–85.
Ross AB, Zangger A, Guiraud SP. Cereal foods are the major source of betaine in the Western diet–analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem. 2014;145:859–65.
Kettunen H, Peuranen S, Tiihonen K, Saarinen M. Intestinal uptake of betaine in vitro and the distribution of methyl groups from betaine, choline, and methionine in the body of broiler chicks. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:269–78.
Rosas-Rodríguez JA, Valenzuela-Soto EM. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: Insights into disease and dysfunction networks. Life Sci. 2021;285.
Figueroa-Soto CG, Valenzuela-Soto EM. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie. 2018;147:89–97.
Schwahn BC, Hafner D, Hohlfeld T, Balkenhol N, Laryea MD, Wendel U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br J Clin Pharmacol. 2003;55:6–13.
Olthof M, Verhoef P. Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab Curr Drug Metab. 2005;6:15–22.
Turck D, Bresson J, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, et al. Safety of betaine as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. Wiley. 2017;15.
Lipiński K, Szramko E, Jeroch H, Matusevičius P. Effects of betaine on energy utilization in growing pigs - a review. Ann Anim Sci. Walter de Gruyter GmbH. 2012;12:201–300.
Ratriyanto A, Mosenthin R. Osmoregulatory function of betaine in alleviating heat stress in poultry. J Anim Physiol Anim Nutr (Berl). 2018;102:1634–50.
Söderlund T, Zhu K, Jutila A, Kinnunen PKJ. Effects of betaine on the structural dynamics of Thermomyces (Humicola) lanuginosa lipase. Colloids Surfaces B Biointerfaces Elsevier. 2002;26:75–83.
Courtenay ES, Capp MW, Anderson CF, Record MT. Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry. 2000;39:4455–71.
Alfieri RR, Cavazzoni A, Petronini PG, Bonelli MA, Caccamo AE, Borghetti AF, et al. Compatible osmolytes modulate the response of porcine endothelial cells to hypertonicity and protect them from apoptosis. J Physiol. 2002;540:499–508.
So P, Wk K. Effects of betaine on biological functions in meat-type ducks exposed to heat stress. Poult Sci. 2017;96:1212–8.
Willingham BD, Ragland TJ, Ormsbee MJ. Betaine supplementation may improve heat tolerance: potential mechanisms in humans. Nutrients. 2020;12:1–14.
Hundahl C, Fago A, Malte H, Weber RE. Allosteric effect of water in fish and human hemoglobins. J Biol Chem. 2003;278:42769–73.
Horio M, Ito A, Matsuoka Y, Moriyama T, Orita Y, Takenaka M, et al. Apoptosis induced by hypertonicity in Madin Darley canine kidney cells: protective effect of betaine. Nephrol Dial Transplant. 2001;16:483–90.
Armstrong LE, Casa DJ, Roti MW, Lee EC, Craig SAS, Sutherland JW, et al. Influence of betaine consumption on strenuous running and sprinting in a hot environment. J strength Cond Res 2008;22:851–60.
Nobari H, Cholewa JM, Castillo-Rodríguez A, Kargarfard M, Pérez-Gómez J. Effects of chronic betaine supplementation on performance in professional young soccer players during a competitive season: a double blind, randomized, placebo-controlled trial. J Int Soc Sports Nutr. BioMed Central Ltd. 2021;18.
Apicella JM, Lee EC, Bailey BL, Saenz C, Anderson JM, Craig SAS, et al. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur J Appl Physiol. 2013;113:793–802.
Nobari H, Cholewa JM, Pérez-Gómez J, Castillo-Rodríguez A. Effects of 14-weeks betaine supplementation on pro-inflammatory cytokines and hematology status in professional youth soccer players during a competition season: a double blind, randomized, placebo-controlled trial. J Int Soc Sports Nutr. 2021;18.
Pryor JL, Craig SAS, Swensen T. Effect of betaine supplementation on cycling sprint performance. J Int Soc Sports Nutr. 2012;9.
Van Every DW, Plotkin DL, Delcastillo K, Cholewa J, Schoenfeld BJ. Betaine Supplementation: A critical review of its efficacy for improving muscle strength, power, and body composition. Strength Cond J Ovid Technologies (Wolters Kluwer Health). 2021;43:53–61.
Moro T, Badiali F, Fabbri I, Paoli A. Betaine supplementation does not improve muscle hypertrophy or strength following 6 weeks of cross-fit training. Nutrients. 2020;12:1–10.
Hoffman JR, Ratamess NA, Kang J, Rashti SL, Faigenbaum AD. Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr. Taylor & Francis. 2009;6:7.
Mahmoud AM, Ali MM. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients. 2019;11.
Bekdash RA. Early life nutrition and mental health: the role of dna methylation. Nutrients. Multidisciplinary Digital Publishing Institute. 2021;3111.
Larkin BP, Glastras SJ, Chen H, Pollock CA, Saad S. DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J. John Wiley & Sons, Ltd. 2018;32:5215–26.
Zhao G, He F, Wu C, Li P, Li N, Deng J, et al. Betaine in inflammation: mechanistic aspects and applications. Front Immunol Front Media S.A. 2018;9:1070.
Li C, Wang Y, Li L, Han Z, Mao S, Wang G. Betaine protects against heat exposure-induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones. 2019;24:453–60.
Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci. 2005;60:1252–64.
Lee EK, Jang EJ, Jung KJ, Kim DH, Yu BP, Chung HY. Betaine attenuates lysophosphatidylcholine-mediated adhesion molecules in aged rat aorta: modulation of the nuclear factor-κB pathway. Exp Gerontol. 2013;48:517–24.
Je JH, Lee JY, Jung KJ, Sung B, Go EK, Yu BP, et al. NF-kappaB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett. 2004;566:183–9.
Kim DH, Kim SM, Lee B, Lee EK, Chung KW, Moon KM, et al. Effect of betaine on hepatic insulin resistance through FOXO1-induced NLRP3 inflammasome. J Nutr Biochem. 2017;45:104–14.
Xia Y, Chen S, Zhu G, Huang R, Yin Y, Ren W. Betaine inhibits interleukin-1β production and release: potential mechanisms. Front Immunol Front Media SA; 2018;9:2670.
Bingül I, Başaran-Küçükgergin C, Aydin AF, Çoban J, Doğan-Ekici I, Doğru-Abbasoğlu S, et al. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ Toxicol Pharmacol. 2016;45:170–8.
Hagar H, Husain S, Fadda LM, Attia NM, Attia MMA, Ali HM. Inhibition of NF-κB and the oxidative stress -dependent caspase-3 apoptotic pathway by betaine supplementation attenuates hepatic injury mediated by cisplatin in rats. Pharmacol Rep. 2019;71:1025–33.
Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients. 2018;10.
Zhang L, Qi Y, Aluo Z, Liu S, Zhang Z, Zhou L. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct Royal Society of Chemistry. 2019;10:216–23.
Chen W, Zhang X, Xu M, Jiang L, Zhou M, Liu W, et al. Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE -/- mice. Eur J Nut. 2021;60:1655–68.
Airaksinen K, Jokkala J, Ahonen I, Auriola S, Kolehmainen M, Hanhineva K, et al. High-fat diet, betaine, and polydextrose induce changes in adipose tissue inflammation and metabolism in C57BL/6J mice. Mol Nutr Food Res. 2018;62:1–33.
Ahmad NA, Raizman M, Weizmann N, Wasek B, Arning E, Bottiglieri T, et al. Betaine attenuates pathology by stimulating lipid oxidation in liver and regulating phospholipid metabolism in brain of methionine-choline-deficient rats. FASEB J. 2019;33:9334–49.
Cholewa JM, Guimarães-Ferreira L, Zanchi NE. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids. 2014;46:1785–93.
Gao X, Wang Y, Randell E, Pedram P, Yi Y, Gulliver W, et al. Higher dietary choline and betaine intakes are associated with better body composition in the adult population of Newfoundland, Canada. PLoS One. Public Lib Sci. 2016;11:e0155403.
Jung GY, Won SB, Kim J, Jeon S, Han A, Kwon YH. Betaine alleviates hypertriglycemia and tau hyperphosphorylation in db/db mice. Toxicol Res. 2013;29:7–14.
Wang L, Chen L, Tan Y, Wei J, Chang Y, Jin T, et al. Betaine supplement alleviates hepatic triglyceride accumulation of apolipoprotein E deficient mice via reducing methylation of peroxisomal proliferator-activated receptor alpha promoter. Lipids Health Dis. 2013;12.
Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol. Baishideng Publishing Group Inc. 2008;14:22.
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr. 2015;113:1835–43.
Ejaz A, Martinez-Guino L, Goldfine AB, Ribas-Aulinas F, De Nigris V, Ribó S, et al. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes. 2016;65:902–12.
Chau MDL, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010;107:12553–8.
Pekkinen J, Olli K, Huotari A, Tiihonen K, Keski-Rahkonen P, Lehtonen M, et al. Betaine supplementation causes increase in carnitine metabolites in the muscle and liver of mice fed a high-fat diet as studied by nontargeted LC-MS metabolomics approach. Mol Nutr Food Res. 2013;57:1959–68.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013 1910. Nature Publishing Group. 2013;19:1252–63.
Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. Am J Physiol Gastrointest Liver Physiol; 2006;290.
Fan CY, Wang MX, Ge CX, Wang X, Li JM, Kong LD. Betaine supplementation protects against high-fructose-induced renal injury in rats. J Nutr Biochem. 2014;25:353–62.
Kang H, Zhang Z, Yu L, Li Y, Liang M, Zhou L. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J Cell Biochem. 2018;119:5676–85.
Metzler-Zebeli BU, Eklund M, Mosenthin R. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. World's Poult Sci J. 2009;65(3):419-42
Hamid H, Pourreza J, Rahimi H. Dietary betaine affect duodenal histology of broilers challenged with a mixed coccidial infection. Pak J Biol Sci PJBS. 2009;12:291–5.
Eklund M, Bauer E, Wamatu J, Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 2005;18:31–48.
Liu W, Yuan Y, Sun C, Balasubramanian B, Zhao Z, An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Anim an Open Access J from MDPI. Multidisciplinary Digital Publishing Institute (MDPI); 2019;9:506.
Alhotan RA, Al Sulaiman AR, Alharthi AS, Abudabos AM. Protective influence of betaine on intestinal health by regulating inflammation and improving barrier function in broilers under heat stress. Poult Sci. 2021;100.
Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Xun J, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–94.
Wang H, Li S, Fang S, Yang X, Feng J. Betaine improves intestinal functions by enhancing digestive enzymes, ameliorating intestinal morphology, and enriching intestinal microbiota in high-salt stressed rats. Nutrients. 2018;10:1–14.
Koistinen VM, Kärkkäinen O, Borewicz K, Zarei I, Jokkala J, Micard V, et al. Contribution of gut microbiota to metabolism of dietary glycine betaine in mice and in vitro colonic fermentation. Microbiome BioMed Central Ltd. 2019;7:1–14.
Chen Q, Wang Y, Jiao F, Shi C, Pei M, Wang L, et al. Betaine inhibits Toll-like receptor 4 responses and restores intestinal microbiota in acute liver failure mice. Sci Rep Nature Publishing Group. 2020;10:1–14.
Chen W, Xu M, Xu M, Wang Y, Zou Q, Xie S, et al. Effects of betaine on nonalcoholic liver disease. Nutr Res Rev. Cambridge University Press; 2021;1–11.
Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front. Pharmacol. 2019. p. 1360.
Zhu W, Buffa JA, Wang Z, Warrier M, Schugar R, Shih DM, et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost. 2018;16:1857–72.
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. NIH Public Access; 2013;19:576.
Ferguson JF. Meat-Loving Microbes. Circ Cardiovasc Genet. Lippincott Williams & WilkinsHagerstown, MD; 2013;6:308–9.
Wang J, Gu X, Yang J, Wei Y, Zhao Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front Cell Infect Microbiol. Front Media S.A. 2019;9:409.
Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83.
Zeisel SH, Da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. NIH Public Access; 2009;67:615.
Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10.
Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, et al. L-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129:373–87.
Simó C, García-Cañas V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020;11(8):6745-76.
Chen S, Jiang P ping, Yu D, Liao G cheng, Wu S ling, Fang A ping, et al. Effects of probiotic supplementation on serum trimethylamine-N-oxide level and gut microbiota composition in young males: a double-blinded randomized controlled trial. Eur J Nutr. 2021;60:747–58.
Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, et al. Effects of probiotic supplementation on trimethylamine-N-oxide plasma levels in hemodialysis patients: a pilot study. Probiotics Antimicrob Proteins. Springer New York LLC; 2019;11:648–54.
Zhu C, Sawrey-Kubicek L, Bardagjy AS, Houts H, Tang X, Sacchi R, et al. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr Res. 2020;78:36–41.
Berge RK, Ramsvik MS, Bohov P, Svardal A, Nordrehaug JE, Rostrup E, et al. Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults - a pilot study. Lipids Health Dis. 2015;14:163.
Delgado-Reyes CV, Wallig MA, Garrow TA. Immunohistochemical detection of betaine-homocysteine S-methyltransferase in human, pig, and rat liver and kidney. Arch Biochem Biophys. 2001;393:184–6.
Lever M, Sizeland PCB, Bason LM, Hayman CM, Robson RA, Chambers ST. Abnormal glycine betaine content of the blood and urine of diabetic and renal patients. Clin Chim Acta. 1994;230:69–79.
Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11.
Qi S, Ouyang X, Wang L, Peng W, Wen J, Dai Y. A pilot metabolic profiling study in serum of patients with chronic kidney disease based on (1) H-NMR-spectroscopy. Clin Transl Sci. 2012;5:379–85.
Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep Nature Publishing Group. 2017;7:1–12.
Hagar H, Al MW. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: role of oxidative stress and caspase-3. Environ Toxicol Pharmacol. 2014;37:803–11.
Van Guldener C, Janssen MJFM, De Meer K, Donker AJM, Stehouwer CDA. Effect of folic acid and betaine on fasting and postmethionine-loading plasma homocysteine and methionine levels in chronic haemodialysis patients. J Intern Med. 1999;245:175–83.
McGregor DO, Dellow WJ, Robson RA, Lever M, George PM, Chambers ST. Betaine supplementation decreases post-methionine hyperhomocysteinemia in chronic renal failure. Kidney Int Elsevier. 2002;61:1040–6.
Wang L, Zhao M, Liu W, Li X, Chu H, Bai Y, et al. Association of betaine with blood pressure in dialysis patients. J Clin Hypertens (Greenwich). 2018;20:388–93.
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Funding
This study was supported by Conselho Nacional de Pesquisa (CNPq) number 200162/2020–9, and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) number E-26/202.524/2019.
Author information
Authors and Affiliations
Contributions
All authors effectively participated in the preparation of this article.
Corresponding author
Ethics declarations
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors do not have any potential conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human oranimal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Foods
Rights and permissions
About this article
Cite this article
Alvarenga, L., Ferreira, M.S., Kemp, J.A. et al. The Role of Betaine in Patients With Chronic Kidney Disease: a Narrative Review. Curr Nutr Rep 11, 395–406 (2022). https://doi.org/10.1007/s13668-022-00426-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-022-00426-z