Abstract
Purpose of Review
The SARS-CoV-2 (COVID-19) outbreak has manifested into a major public health concern across the globe, affecting particularly the most vulnerable population groups. Currently, there are various clinical trials being conducted to develop effective treatments. It is estimated that it could take one or more years before these drugs pass all safety tests and concrete results with regard to their effectiveness become available. In addition, despite the recent development of vaccines (licensed for use under conditional licenses) and the commencement of COVID-19 vaccination programs in several countries, there is still a need for safe and novel strategies that may reduce the symptomatology and/or prevent the severe complications associated with COVID-19. Natural compounds previously shown to have antiviral potential should be thoroughly considered and investigated for use in prophylactic treatment of COVID-19 due to their availability and safety.
Recent Findings
The current narrative review investigates whether there is evidence in the literature that supplementation with dietary minerals and vitamins may have a role in preventing infection with SARS-CoV-2 or in reducing COVID-19 symptomatology and disease progression. The current evidence from the literature supports that zinc and vitamin C have a potential in reducing the inflammatory response associated with SARS-CoV-2 while folate and vitamin D may have a role in antagonizing the entry of SARs-CoV-2 virus in host calls.
Summary
Thus, further research should be conducted that could lead to the development of nutritional supplements involving natural and widely available compounds such as zinc, folate, vitamin C, and vitamin D. The latter could be an effective, safe, and inexpensive way to either prevent infection with SARS-CoV-2 and/or lessen the burden of COVID-19 disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) is a novel beta coronavirus that is responsible for the outbreak that emerged in late 2019. The first cases of COVID-19 are believed to have originated in Wuhan, Hubei Province, China, in December 2019 with a cluster of patients presenting with Acute Respiratory Distress Syndrome (ARDS) [1••]. By January, the number of cases was rising rapidly, spreading across China and starting to spread across different countries. By March 2020, only 3 months following the initial cases, the World Health Organization (WHO) announced its pandemic status. According to a situation report from the World Health Organization (WHO), as of January 19, 2021, COVID-19 was responsible for a total of 93,956,883 confirmed cases and 2,029,084 deaths [2].
SARS-CoV-2 is an enveloped positive sense single stranded RNA virus targeting the human respiratory system. Patients infected with the virus present with a vast number of symptoms including but not limited to fever, non-productive cough, fatigue, sore throat, headache, and shortness of breath [1••]. Additional symptoms have started to emerge as COVID-19 continues to spread, including gastrointestinal and cardiovascular symptoms [3]. The transmission is thought to occur from person to person (i.e., through close contact of an uninfected individual with an infected individual) and/or possibly through contact of an uninfected individual with infected droplets on surfaces [1••]. Even though SARS-CoV-2 has infected every single population group (across age, race, and gender), there are certain vulnerable population groups that present more severe symptomatology including individuals over the age of 70 years and those with existing comorbid disorders [4•].
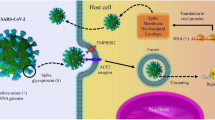
Recently, it has been discovered that entry of SARS-Cov-2 into host tissue within the lungs is mediated through docking of the viral envelope to angiotensin converting enzyme 2 (ACE-2) and CD209L. Viral docking of SARS-CoV-2 is facilitated through two key cell surface spike proteins, S1 and S2. Viral surface protein S1 binds uniquely to ACE-2 receptors, allowing protein S2 to mediate cellular endocytosis of the virus. It has also been noted that ACE-2 is also expressed on lymphocytes, further highlighting COVID-19 is not an infection limited to the lung tissue but rather has an infective potential beyond respiratory tissue [5•]. Furthermore, it has been observed that expression of ACE-2 is greater in patients with certain comorbidities including hypertension, diabetes mellitus, and coronary and cerebrovascular disease. As such, the functional activity of ACE-2 is associated with the severity of symptoms of the COVID-19 infection [6].
Efforts for the development of a vaccine against the viral pathogen SARS-CoV-2 were mediated through various organizations throughout the world. Throughout 2020, 58 different vaccines against SAR-CoV-2 have been developed in clinical trials [7, 8]. However, the leading vaccines currently available are the ones manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca [7, 8]. Currently, all three vaccines have been selected by governments around the world for widespread vaccination programs. The leading vaccines are using the mRNA technology which is a powerful immunomodulatory alternative to conventional attenuated vaccine approaches [7, 8]. Though the use of mRNA vaccines has so far been limited, their low cost and rapid development have been proposed as a powerful platform for SARS-CoV-2 vaccination. The most widely employed vaccine has been the one developed by Pfizer-BioNTech, and this vaccine named BNT162b2 demonstrated 95% efficacy in preventing symptomatic and PCR-confirmed COVID-19 infection (CI: 90.3–97.6%) in a randomized double-blind, placebo-controlled phase II/III trial [9••]. As such, this vaccine has been employed under emergency authorization by the FDA for use within the USA [10]. On the other hand, the Moderna vaccine (mRNA-1273) has demonstrated a 94.1% efficacy (CI: 89.3–96.8% with interim data [11••]), and results from AstraZeneca’s ChAd0x1 nCoV-19 vaccine have demonstrated 90.0% efficacy (CI: 67.4–97.0%) [12, 13].
Currently, there is no specific treatment regimen for COVID-19. The current treatment options for patients include supportive measures (antibiotics, high flow oxygen, and proning) and treating underlying conditions. Several ongoing clinical trials have been approved by the Clinical Trial Agencies in testing potential candidate drugs for the management of COVID-19 [14]. These therapeutic agents had previously shown antiviral properties (through in vitro, in vivo, and clinical studies) of the drug against other viruses such as MERS-CoV, human immunodeficiency virus (HIV), and hepatitis B virus (HBV) [14]. These therapeutic agents under investigation include hydroxychloroquine, remdesivir, favipiravir, lopinariv/ritonavir, tocilizumab, convalescent plasma, and immunoglobulins [14]. The first preliminary results from these trials showed mixed efficacy with conflicting results. Clinical trials are still underway; however, currently there is insufficient evidence to recommend the use of any one of these agents. WHO recommends against the use of Remdesivir in hospitalized patients as there is no evidence that it improves patient outcomes by reducing symptoms or decreasing mortality [14]. Initially, the evidence for hydroxychloroquine/chloroquine were promising; however, current evidence demonstrates no added benefit [15]. Lopinavir/ritonavir has also shown little to no effect among hospitalized patients [16]. There are mixed results with tocilizumab, an IL-6 inhibitor. The National Institute of Health recommends against its use due to low evidence [17]; however, a phase 3 randomized control trial found that it could reduce the need for mechanical ventilation, but there was no overall effect in terms of reduced mortality [18]. Monoclonal antibodies (casirivimab and imdevimab) have been used in patients presenting to the emergency department as these have been shown to reduce viral load from baseline [19]. Intravenous immunoglobulins used as an adjunctive treatment have been shown to possibly reduce the need for mechanical ventilation and have resulted in a reduced mortality [20•]. Furthermore, there is some evidence that convalescent plasma reduces mortality, increases viral clearance, and improves symptoms in patients [21]. Overall, the results from drugs tested for the management of COVID-19 remain preliminary, and further randomized controlled trials need to be conducted to derive conclusive results [22].
Thus, there is an urgent need for alternative, more preventative strategies to reduce the spread of the SARS-CoV-2 virus and to prevent the complications of COVID-19. The aim of the current review is to investigate whether any readily available nutrients (minerals and vitamins) with known safety may have a role in preventing infection with SARS-CoV-2 or in reducing disease progression of COVID-19.
Methods
Relevant articles to include in the review were identified by searching PubMed database using the following search terms: COVID-19 OR SARS-CoV-2 OR CoronaVirus AND (Folate OR Zinc OR Vitamin D OR Vitamin C OR vitamin OR micronutrients).
Only studies published in English after the year 2000 were included with the majority of articles taken from 2020. The reference lists of relevant articles were also reviewed in order to identify additional appropriate articles pertaining to the search criteria. The abstracts of all derived papers were evaluated by two reviewers (NUP and TOT), and papers not relevant to the review’s aim were excluded. We further examined studies that specifically mentioned SARS-CoV-2 in relation to mortality, ventilation use, symptom reduction, and complications.
The Role of Dietary Minerals and Vitamins May Have a Role in Preventing Infection with SARS-CoV-2 or in Reducing COVID-19 Symptomatology and Disease Progression
Zinc
Zinc is a fundamental dietary trace element which is normally present at low intracellular concentrations in the blood. Sources of zinc can be found in lamb, beef, chicken, oysters and lobsters, black rice, mushroom, celery, legumes, lentils, and nuts [23].
Zinc is required for a variety of essential biochemical processes ranging from mediating appropriate protein folding, regulating transcription, and acting as both a cofactor and a secondary messenger in vital cellular processes [24]. Zinc is the structural component of more than 750 zinc-finger transcription factors [23, 24]. Some studies have suggested zinc’s possible role as a potent inhibitor against replication of the SARS-coronavirus, hepatitis C virus, and H1N1 influenza virus by inhibiting zinc oxide and zinc salt [25, 26]. The particular mechanism behind its inhibitory effect is still speculative; however, potential sites of action include the proteolytic processing of replicase polyproteins, viral RNA-dependent RNA polymerase (RdRp), and cellular cofactors involved in replication [24,25,26].
Zinc and Inhibition of Viral Replication
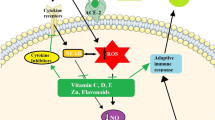
Several studies have noted zinc’s role in modulating antibacterial immunity and regulating the inflammatory response by inhibiting NF-κB, TNF-α, and IFN-γ signaling and modulating proliferation, differentiation, and maturation of lymphocytes to lessen the cytokine storm [27•]. In addition, studies have provided evidence showing that zinc may have the ability to decrease the activity of ACE-2, the receptor implicated in SARS-CoV-2 and a type I integral membrane bound protein found in epithelial cells of the heart, lung, intestines, and kidney [20•]. The viral binding of SARS-CoV-2 to the host cell is mediated through four essential structural proteins, one of which being the spike protein [28•]. Composed of two subunits (S1 and S2), the spike protein uniquely binds to the respiratory epithelium expressing ACE-2 and CD203L [28•]. An interaction exists between this S glycoprotein on SARS-CoV-2 and the ACE-2 receptor [29•]. Studies have shown that exogenous zinc blocks the ability of ACE-2 to metabolize substrate, suggesting zinc’s possible ability to inhibit the interaction between SARS-CoV-2 S protein and ACE-2 [20•].
It has been suggested that zinc can inhibit coronovirus replication by the inhibition of RNA synthesis [25]. Cell culture studies have found that zinc can efficiently inhibit both the SARS-CoV and Nidovirus replication transcription complexes (RTC) [25]. In addition, antiviral effects of zinc have been reported in H1N1 influenza [30], respiratory syncytial virus [31] hepatitis C virus [32], and rhinovirus [27]. In addition, in developing countries, zinc has been used to reduce the prevalence of pneumonia in young children [33], and zinc deficiency has been correlated with increased susceptibility to pneumonia among the elderly [27]. Since SARS-CoV-2 shares similarities with some of these viruses; in theory, zinc could be effective against Sars-CoV-2, and therefore it should be considered as a potential therapeutic agent in antiviral therapy for SARS-CoV-2 [20•].
Zinc and the Inflammatory Response
COVID-19 is associated with the activation of the inflammatory cascade since infected patients present elevated cytokines (IFN-γ, interleukin-2, 6, 7, and TNF), macrophages, and C-reactive protein, all of which are inflammatory mediators [34]. These cytokines are essential for both innate and adaptive immunity against viral infections. Zinc has been strongly implicated in immune regulation through mediation of inflammation, i.e., increased production of pro-inflammatory cytokines that have immunomodulatory, anti-proliferative, and antiviral properties such as IL-1, IL-6, and TNF-α [35, 36•].
One of the products of the inflammatory cascade is the production of excess mucus within the respiratory tract. Elevated levels of zinc found within the mucosa of both the oral and nasal respiratory tract have been found to be positively correlated with a reduction in symptoms of Rhinovirus, a virus which is transmitted in a similar fashion to SARS-CoV-2 and leads to the development of similar symptoms [23]. Supplementation of zinc has the possible benefit of improving the membrane barrier function and mucociliary clearance of the epithelial cells in the respiratory tract [27•]. This is done by increasing tight junction proteins and increasing ciliary length and beat frequency, thereby reducing the risk of bacterial co-infections, particularly those caused by Streptococcus pneumaoniae pneumoniae [27•]. Zinc deficiency has also been shown to up-regulate acute phase response-related genes, increased susceptibility to systemic inflammation, and sepsis-induced organ damage [27•].
Zinc and the Immune System
Infection recovery and viral clearance require the activation of the host’s immune response. The natural process of aging is associated with deterioration of both the innate and adaptive immune system. This includes a declining T cell function due to thymic involution, reduced output of immune cells from the bone marrow and thus reduced capacity to respond to new antigens, age-associated inflammation with increased serum concentration of inflammatory mediators, and poor micronutrient status. Naturally, this predisposes the elderly to pulmonary disease [29•, 37, 38•]. Immunonutrition, i.e., modulating the immune response through supplementation of essential vitamins could be an easy, preventative step of reinforcing the immune system and thus reducing the risk among the elderly against infection of invading pathogens. Despite the lack of sufficient evidence with regard to the direct role of zinc on SARS-CoV-2, evidence in the literature supports that zinc is important in supporting our immune system in the fight against bacterial and viral infections [39].
Effects of Zinc Deficiency and Supplementation
Zinc deficiency has been well noted to depress both innate and adaptive immune responses with reduced production of antibodies that lead to increased susceptibility to both bacterial and viral infections [36•, 40]. Chronic deficiency of zinc has been found to increase the susceptibility of many inflammatory, metabolic, neurodegenerative, and immune-mediated diseases such as diabetes, obesity, and cardiovascular disease due to prolonged activation of pro-inflammatory cytokines and oxidative stress [27•]. Individuals with comorbidities, especially those with diabetes and cardiovascular disease, are at a particularly higher risk for developing COVID-19 with more severe symptomatology and higher mortality [27•].
Zinc deficiency has been shown to be associated with low lymphocyte counts and impaired T and B lymphocyte functions [41]. Biochemically, this depressed adaptive immune outcome results in impaired gene expression, prolonging infection duration and symptomatology in patients [41]. Genetic conditions such as Epidermodysplasia verruciformis result in low levels of zinc within cells, which has been observed to increase sensitivity to cutaneous viral infections. An interesting study has shown that zinc supplementation resulted in a higher proportion of CD4+CD3+ cells in peripheral blood, with enhanced T-cell-mediated immunity in supplement-treated children compared to control [42].
It should also be noted that there is a global prevalence of zinc insufficiency which is estimated to be 20%, and this insufficiency is very common among elderly individuals [43]. A study has shown that the consumption of 45 mg of zinc per day reduced the incidence infections in elderly individuals (55–87 years of age) [40]. Therefore, zinc supplementation (with a recommended dose of 50 mg per day) could have a preventive or therapeutic potential for managing COVID-19.
Zinc and Hydroxychloroquine/Chloroquine
There is growing evidence from in vitro studies and recent ongoing clinical trials for the role of chloroquine (CQ) and its derivative and less toxic hydroxychloroquine (HCQ) as potential therapeutic agents against SARS-CoV-2 [22]. Both of these drugs are well noted for their anti-malarial and immunomodulatory effects against other viruses such as the Zika virus and the Ebola virus. CQ and HCQ are both described as metal ionophores, drugs that have metal binding domains and thus act as cation transporters for both zinc (Zn2+) and copper (Cu2+) [44•]. These metal ionophores have been shown to have both antiviral and anti-cancer properties through acting as weak bases, binding to excess zinc in viral-infected tissues and directly interfering with the synthesis of both viral DNA-dependent DNA polymerase and RNA-dependent RNA polymerase [44•]. They are known to accumulate within intracellular vesicles of endosomes, lysosomes, and Golgi bodies, increasing the pH within these organelles [45]. Thus, CQ/HCQ are thought to have direct antiviral effects by inhibiting pH-dependent steps in the SARS-CoV-2 replication process [45]. CQ/HCQ also have indirect antiviral effects by interfering with the delivery of viral particles into host cells since SARS-CoV-2 requires the acidification, lowered pH, of endosomes for proper functioning [45]. The bioavailability of CQ and HCQ depends on their protonation with zinc ions. CQ/HCQ has the ability to trigger the uptake of extracellular zinc into intracellular lysosomes followed by the inhibition of the RNA-dependent RNA polymerase halting the replication of SARS-CoV-2 [44•, 45]. Thus, the supplementation of zinc alongside CQ/HCQ therapy could have a synergistic therapeutic effect against SARS-CoV-2 and possibly be more effective in reducing morbidity and mortality as compared to CQ/HCQ monotherapy alone [45]. Skalny et al. hypothesized the use of zinc supplementation without the intake of CQ to produce similar positive effects in the absence of the adverse side effects seen in CQ treatment; however, this is yet to be tested [27•].
Folate
Folate, otherwise known as vitamin B9, and its subsequent derivatives such as folic acid are found widely within vegetables, in particular dark green leafy vegetables, fruit, nuts, meat products, and dairy products [46]. Folate deficiency is commonly observed in specific patient populations, e.g., pregnant women or patients with chronic conditions or patients who are over the age of 65 [46].
Adequate dietary levels of folate are essential for normal nucleic acid synthesis, methionine regulation, and essential oxidative and reductive cellular processes [47]. Folate deficiency has been implicated in carcinogenesis resulting from poor transcriptional regulation and inadequate DNA methylation. Most importantly, however, folate plays a crucial function in carbon metabolism, and therefore folate deficiency has severe metabolic complications. Evidence in the literature supports that folate deficiency impairs the body’s ability to mount an effective immune response [47]. As such its importance, many foods such as bread are fortified by the addition of folic acid to prevent deficiencies in the general population [46].
Folic Acid, Furin, and SARS-CoV-2
As previously described, the viral binding of SARS-CoV-2 is mediated through four essential structural proteins, one of which being the spike protein [28•]. Upon docking, the spike proteins are known to undergo proteolytic activation mediated through furin motifs present on the cell surface. Furin has been implicated as a key proteolytic enzyme required for numerous pathological processes such as neoplasia and bacterial toxin activation [28•]. In addition, furin has been found to have a role in the activation and binding of viral pathogens, such as ones belonging to the herpesvirus and the coronavirus families. Thus, inhibition of furin activity has been proposed as a means of inhibiting the pathogenesis of viral infection. Folic acid has been suggested as a novel agent to directly antagonize the activity of furin and thus impede the biological docking and binding of SARS-CoV-2 to its target cells [48].
One study has demonstrated folate’s ability to consume binding sites through in silico molecular dynamic simulations [28•]. Interactions between furin and folate compounds such as folic acid and folinic acid were examined using simulated intermolecular interactions. This study concluded that furin conferred a high degree of steric hindrance [28•]. Thus, consuming furin binding sites directly antagonizes the access of the SARS-CoV-2 spike protein to furin, thereby halting viral binding and cellular pathogenesis.
Levels of Folic Acid in COVID-19 Patients
Interestingly, in patient cohorts infected with SARS-CoV-2 in Israel, the most severely infected patients showed to have significantly lower levels of serum folic acid. As such, due to the non-immunogenic and benign nature of folic acid, it could have a potential role in being used as a nutritional supplement to prevent infection and disease severity of COVID-19 [28•]. However, further studies should be carried out to investigate whether this is a common observation in other COVID-19 patient cohorts.
Vitamin C
Another agent, known to play a significant role in the immunological function in humans is vitamin C, also termed ascorbic acid, naturally found in fruits and vegetables. A substantial amount of research over the last century has implicated vitamin C as a crucial player in the maintenance of a healthy immune system and acting as a prophylaxis against illness [49]. Its pleiotropic biochemical nature allows it to function as a potent water-soluble primary antioxidant which protects tissues from cellular oxidative stress (produced as a by-product from normal cellular metabolism and from exposure to toxins in the environment) [50]. Vitamin C also regenerates cellular and membrane antioxidants such as glutathione and vitamin E (tocopherol) [51]. The deficiency of vitamin C has been well noted to lead to a decreased immune function and increased vulnerability and susceptibility to infections. The supplementation of vitamin C has been widely used for the prevention and treatment of respiratory infections [52•].
Vitamin C and the Immune System
Although, not as clearly understood, vitamin C has also been found to have a role in the adaptive immune response by enhancing the development, proliferation, and maturation of lymphocytes [51]. The role vitamin C plays for lymphocytes is still speculative; however, it has been suggested to have antioxidant protective effects, enhanced antibody production, and the enhancement of natural killer T cell activity [50, 53].
Vitamin C has been shown to play a role in each step of the inflammatory cascade of the innate immune system through preventing the entrance of pathogens, migration of leukocytes to the site of infection, engulfing the invading pathogen, to apoptosis and clearance of macrophages following the resolution of the infection [50, 53]. Vitamin C is accumulated within epidermal and dermal cells contributing to the innate immune system by maintaining the integrity of the epithelial barrier against foreign invaders and environmental toxins and directly scavenging against free radicals on the skin and mucosal surfaces [50, 54]. Studies have notably demonstrated vitamin C’s antioxidant effect within lung epithelioid cells by reducing oxidative stress and subsequently inflammation.
Acute respiratory distress syndrome (ARDS) is a common cause of death among patients infected with SARS-CoV-2, characterized by a cytokine storm, the rapid release of free radicals and cytokines into the periphery leading to the activation of capillary endothelial cells, neutrophil infiltration, and increased oxidative stress [55•]. This results in cellular damage, organ failure, and finally death among these patients. Several studies have identified the early use of high-dose vitamin C as a potential therapeutic role in reducing the cytokine storm seen by its antioxidant effect [55•].
Effects of High-Dose Vitamin C Supplementation on Respiratory Infections and COVID-19
Several studies have been carried out to investigate whether vitamin C supplementation can reduce both the severity and duration of upper respiratory tract infections in some studies [29•, 36•].
One meta-analysis study comprising 29 randomized controlled trials containing 11,306 participants demonstrated that regular intake of 1 g of vitamin C did not provide prophylactic protection for upper respiratory tract infections (URTIs). However, the supplementation reduced the duration of the infection and helped alleviate the symptoms associated with URTIs [56].
The CITRIS-ALI trial was a randomized, double-blind, placebo-controlled, multicenter trial conducted in the USA. The objective of the study was to determine the effect of intravenous vitamin C infusion on organ failure scores and biological markers of inflammation and vascular injury in patients with sepsis and ARDS. In this study, a 96-h infusion of vitamin C did not significantly improve organ dysfunction scores or alter markers of inflammation and vascular injury in the experimental group when compared with the placebo group. The researchers proposed that further research is needed to evaluate the potential role of vitamin C for other outcomes in sepsis and ARDS [57].
Furthermore, a meta-analysis by Cheng et al. [55•] demonstrated that in 12 trials with 1766 patients, vitamin C reduced the length of ICU stay on average by 8%. The meta-analysis study has shown that even though in healthy individuals, 0.1 g/day of vitamin C was needed to maintain a normal plasma concentration, much higher doses (1–4 g/day) were required to increase the plasma vitamin C concentration to increase to the normal level in critically ill patients [55•].
The clinical trial ‘Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia’ (Identifier: NCT04264533) [58, 59] was conducted in Wuhan, China in March 2020. The aim of this trial was to investigate the use of vitamin C infusion for the treatment of severe Sars-CoV-2-infected pneumonia. In this trial, the investigators treated 140 patients with a placebo control or intravenous vitamin C at a dose of 24 g/day for 7 days. The high-dose intravenous vitamin C improved arterial oxygenation, likely due to improved pulmonary ventilation function, reduced the 28-day mortality rate, and decreased the levels of IL-6 in comparison to the placebo group [58, 59].
In addition, a randomized, quadruple clinical trial (Early Infusion of Vitamin C for Treatment of Novel COVID-19 Acute Lung Injury (EVICT-CORONA-ALI) (Identifier: NCT04344184) started in October 2020 [60] to investigate if a 72-h intravenous vitamin C infusion protocol (100 mg/kg every 8 h) in patients with hypoxemia and suspected COVID-19 will reduce the lung injury caused by the SARS-CoV-2. This is a phase II trial and will use 200 patients and will take place in Virginia, USA. The study is expected to be completed by May 2021 [60].
If proven efficacious, intravenous vitamin C would offer a safe and inexpensive therapy against COVID-19. The immunosuppressive function of high-dose vitamin C can potentially be used to control the unrestrained and excessive immunological reaction associated with the pathogenesis of COVID-19.
Vitamin D
1,25-Dihydroxycholecalciferol, the active form of vitamin D, is prominently known for its role in maintaining skeletal homeostasis by regulating calcium and phosphate metabolism in bone. Vitamin D has 2 prominent forms and several metabolites: Vitamins D2 and D3 also known as ergocalciferol and cholecalciferol respectively. Vitamin D3 is liberated within the skin in response to UVB radiation, though it can be obtained from diet mainly through animal sources such as fatty fish, egg, and dairy [61]. Vitamin D2 is typically obtained through plant sources. Though few diets naturally provide the required quantity of vitamin D, it is primarily obtained through fortification of foods [61].
Vitamin D and the Immune System
Vitamin D’s possible antiviral effects have been suggested through both epidemiological findings of enhanced susceptibility and severity of respiratory infections due to vitamin D deficiency. Supplementation with vitamin D has been associated with enhanced recovery and protective effects against respiratory infections [62]. Though the mechanism of Vitamin D’s antiviral effects is still speculative, cell culture experiments have provided some insight into its possible mechanism involving Vitamin D’s ability to up-regulate anti-microbial peptides which are known to be among the first line of defense and have been shown to be able to kill enveloped viruses [63].
Vitamin D has also been linked to contributing to the innate and adaptive immune response as an immunomodulator, targeting both innate immune cells (monocytes, macrophages, dendritic cells) and adaptive immune cells (B and T lymphocytes) [64]. The deficiency of Vitamin D has been well noted in conditions such as rickets within children and osteomalacia in adults causing softening of the bone due to improper mineralization. Vitamin D deficiency has also been linked to higher incidence of chronic infections, autoimmune, and immune-mediated conditions [64]. Vitamin D’s role as an immune regulator was further enhanced by the discovery of immune cells (antigen presenting cells, B cells, and T cells) expressing both vitamin D receptors and vitamin D metabolizing enzymes and their ability to synthesize the active form of vitamin D [65].
Vitamin D and ACE-2
Expression of ACE-2 is directly associated with vitamin D activity; vitamin D has been noted to be a potent down-regulator of ACE-2 [66]. Evidence has shown that deficiency in vitamin D results in an increase in expression of ACE-2 throughout the respiratory epithelium and subsequent increased activity of RAAS [67•]. Thus, supplementation of vitamin D is suspected to reduce viral docking potential through reduction in the expression of ACE-2 throughout the [68]. This is further supported through the potent antiviral features of vitamin D against other respiratory infections such as influenza [66].
Vitamin D has a possible role in attenuating the symptoms of COVID-19 through disrupting the interaction between the virus and target cells but also via enhancing the host’s immunological potential. The exact molecular mechanism through which vitamin D reduces ACE-2 expression is not well established, though it is proposed that it alters the transcriptional activity of ACE-2, thereby reducing the expressed levels of the receptor [69•]. The functional activity of vitamin D, as a steroid hormone, has further been implicated on down-regulating cytokine activity, a fundamental process in COVID-19 pathogenesis which results in respiratory distress [70]. In addition, vitamin D deficiency has been found to be associated with increased airway hypersensitivity, most notably observed in asthmatic patient cohorts [71]. This can be attributed to vitamin D’s ability to attenuate an immunogenic potential within the airways by suppressing the release of cytokines from bronchial smooth muscle cells [71].
Association Between Vitamin D Levels and COVID-19
Increased mortality due to COVID-19 has been observed in groups who have reduced levels of vitamin D [72]. The level of vitamin D in a patient is strongly associated with a variety of other factors, most notably sunlight exposure, nutrition, and physical activity, all of which are independent promoters of immunological function [72]. Increased physical activity can furthermore promote vitamin D synthesis by inadvertently increasing outdoor sunlight exposure [72]. UV-B exposure is required to convert 7-dehydrocholesterol to provitamin D3 which isomerizes into biologically active vitamin D3 [73]. It has been observed that northern latitude nations who on average receive lower hours of sunlight exposure, as a result of seasonal and geographical reasons, have a higher COVID-19 infection rate than those which are more central to the equator [74]. This is most notably seen in populations above the 35 parallel who throughout winter do not receive adequate sunlight exposure to maintain a normal vitamin D status [74].
In the past few months, it has become apparent that older individuals who have a greater risk of developing severe COVID-19 may also present with lower levels of vitamin D when compared to younger adults [75•]. Panrese and Shahini (2020) [76•] have proposed that the very high COVID-19 mortality rate of 11.9% in Italy could be theoretically associated with the reported chronic vitamin D deficiency in the population, especially in the winter season. Nevertheless, this suggestion is based on results from an ecological study which is considered of low level of evidence, and therefore studies of a higher level of evidence (e.g., randomized control trials) should be carefully designed to investigate the association between vitamin D deficiency and COVID-19 morbidity and mortality [76•]. Hribar et al. [75•] examined the relationship between vitamin D, Parkinson disease (PD), and COVID-19 and concluded that the daily supplementation of 2000–5000 IU/day of vitamin D3 in older adults with PD has the potential to slow the progression of PD while also potentially reducing the risk of infection with SARS-CoV-2 [75•].
With such urgency for treatment and vaccination for COVID-19, vitamin D may provide a novel, low-cost, and safe means through which COVID-19 pathogenicity can be controlled [68]. Vitamin D has furthermore proved to be a safe adjuvant therapy which can be provided through dietary supplementation [67•]. Since the expression of ACE-2 in the lungs is dependent on the levels of vitamin D, the latter may pose an important function in preventing and managing COVID-19 [77].
Conclusion
COVID-19 continues to be a major health concern and is currently responsible for a total of 93,956,883 confirmed cases and 2,029,084 deaths, and the number of cases is expected to increase even further [2]. Despite the commencement or expected commencement of vaccination programs with the most promising vaccines in many countries, there is an urgent need for alternative, more preventative strategies to reduce the spread of the SARS-CoV-2 virus and to prevent the complications of COVID-19.
This review has highlighted the possible role the supplementation with nutrients (vitamins and trace elements) may have on improving disease outcomes of COVID-19. The literature supports that zinc has a potential role of being a potent inhibitor against viral replication processes through potential sites of actions in the proteolytic processing. Furthermore, folic acid has been suggested as a potential means of halting viral infection by directly antagonizing furin binding sites required for spike protein mediated docking of COVID-19. In addition, vitamin C is a potent antioxidant that plays a role in facilitating each step of the inflammatory cascade of the innate immune system. It has a large immunomodulatory potential in controlling the deleterious inflammatory effects seen in the SARS-CoV-2 infection. Finally, vitamin D supplementation has demonstrated to be an effective means through which expression of ACE-2 can be down-regulated to reduce infective potential.
It should be noted however that the current literature on the role of micronutrients in reducing the morbidity of COVID-19 is currently limited due to the lack of well-designed clinical trials that investigate micronutrient efficacy, dosing, route, and timing in reducing COVID-19 symptomology and morbidity. Due to this, a narrative rather than a systematic review was conducted.
Overall, micronutrients such as zinc, folate, vitamin C, and vitamin D could be used as a strategy to prevent infection of SARS-CoV-2 or as a novel therapy by being used with other pharmacological agents in the management of COVID-19. Supplementation with the nutrients could be a potential source of providing a safe, effective, and cost-effective means of reducing risk of infection, symptom severity, and duration of recovery with minimal adverse effects. Future studies should look further into the potential therapeutic dosages required and combination therapies of these novel agents.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rothan H, Byrareddy S. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. https://doi.org/10.1016/j.jaut.2020.102433This review provides a thorough description of the epidemiology and pathogenesis of SARS-CoV-2, accounting for its origin, symptomology, transmission, and therapeutic targets.
World Health Organisation Coronavirus Disease (COVID-19) Dashboard. 2021. Available at: https://covid19.who.int/. Accessed on January 19, 2021.
Clerkin K, Fried J, Raikhelkar J, Sayer G, Griffin J, Masoumi A, et al. COVID-19 and Cardiovascular Disease. Circulation. 2020;141(20):1648–55. https://doi.org/10.1161/CIRCULATIONAHA.120.046941.
Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–9. https://doi.org/10.1007/s10096-020-03874-zThis review provides a comprehensive understanding of the various features behind COVID-19: underlying genetics, epidemiology, clinical manifestations, treatment, and prognosis.
Silberstein M. Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19? Med Hypotheses. 2020;140:109767. https://doi.org/10.1016/j.mehy.2020.109767This article provides an insight into the role of Vitamin D in the modulation of cytokine production and thus control of COVID-19 clinical features.
Tian Y, Rong L. Letter: Covid-19, and vitamin D. Authors' reply. Aliment Pharmacol Ther. 2020;51(10):995–6. https://doi.org/10.1111/apt.15764.
European Medicines Agency. Treatments and vaccines for Covid-19. 2021. Available on https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19?utm_source=phpList&utm_medium=email&utm_campaign=Covi. Accessed on January 22, 2021.
Locht C. Vaccines against COVID-19. Anaesth Crit Care Pain Med. 2020;39(6):703–5. https://doi.org/10.1016/j.accpm.2020.10.006.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577This article examines the safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. The researchers report that a two-dose regimen conferred 95% protection in individuals 16 years or older.
Oliver S, Gargano J, Marin M, Wallace M, Curran K, Chamberland M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–4.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. N Engl J Med. 2020:NEJMoa2035389. https://doi.org/10.1056/NEJMoa2035389This article examines the safety and efficacy of the mRNA-1273 SARS-CoV-2 vaccine. They found that a two-dose regimen conferred 94.1% protection in persons at high risk for SARS-CoV-2 infection or its complications.
Knoll M, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–4.
Voysey M, Clemens S, Madhi S, Weckx L, Folegatti P, Aley P, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.4.
Rochwerg B, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. https://doi.org/10.1136/bmj.m3379.
Hernandez A, Roman Y, Pasupuleti V, Barboza J, White C. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19. Ann Intern Med. 2020;173(4):287–96.
Alhumaid S, Al Mutair A, Al Alawi Z, Alhmeed N, Zaidi ARZ, Tobaiqy M. Efficacy and safety of lopinavir/ritonavir for treatment of COVID-19: a systematic review and meta-analysis. Trop Med Infect Dis. 2020;5(4):–180.
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2020 Apr 27:ciaa478. https://doi.org/10.1093/cid/ciaa478.
Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2020;S1198-743X(20):30690-X.
Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2020;384(3):238–51. https://doi.org/10.1056/NEJMoa2035002.
McPherson S, Keunen J, Bird A, Chew E, van Kuijk F. Investigate oral zinc as a prophylactic treatment for those at risk for COVID-19. Am J Ophthalmol. 2020;216:A5–6. https://doi.org/10.1016/j.ajo.2020.04.028This review looks into the potential use of zinc as a safe and widely used prophylactic treatment option against COVID-19. Zinc has been suggested to have anti-viral properties inhibiting the vital interaction between SARS-CoV-2 S glycoprotein and the ACE2 enzyme essential for the replication of SARS-CoV-2.
Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2020;93(2):1111–8. https://doi.org/10.1002/jmv.26408.
Esposito S, Noviello S, Pagliano P. Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Infez Med. 2020;28(2):198–211.
Razzaque M. COVID-19 Pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J Exp Med. 2020;251(3):175–81. https://doi.org/10.20944/preprints202004.0006.v1.
Lambert S, Jolma A, Campitelli L, Das P, Yin Y, Albu M, et al. The human transcription factors. Cell. 2018;172(4):650–65. https://doi.org/10.1016/j.cell.2018.01.029.
te Velthuis A, van den Worm S, Sims A, Baric R, Snijder E, van Hemert M. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. https://doi.org/10.1371/journal.ppat.1001176.
Lanke K, Krenn B, Melchers W, Seipelt J, van Kuppeveld F. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J Gen Virol. 2007;88(4):1206–17. https://doi.org/10.1099/vir.0.82634-0.
Skalny A, Rink L, Ajsuvakova O, Aschner M, Gritsenko V, Alekseenko S, et al. Zinc and respiratory tract infections: perspectives for COVID-19 (Review). Int J Mol Med. 2020;46(1):17–26. https://doi.org/10.3892/ijmm.2020.4575This review looks into zinc’s possible role as an adjuvant therapy against COVID-19 through its protective role in reducing inflammation, lessening the cytochrome storm, improving the membrane barrier function and mucociliary clearance of the epithelial cells in the respiratory tract.
Sheybani Z, Dokoohaki MH, Negahdaripour M, Dehdashti M, Zolghadr H, Moghadami M, et al. The role of folic acid in the management of respiratory disease caused by COVID-19. 2020. https://doi.org/10.26434/chemrxiv.12034980.v1This review discusses the structure of the viral surface spike proteins which SARS-CoV-2 utilises to gain entry into the target respiratory cells. Highlighting the potential of folic acid to be a novel inhibitor of the surface enzyme Furin, a required enzyme for proteolytic activation of surface proteins facilitating viral entry.
Calder P. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020;3(1):74–92. https://doi.org/10.1136/bmjnph-2020-000085This review discusses the importance of several vitamins and minerals in supporting the immune system and fight against infections and more specifically in reducing the cytokine storm characteristic of COVID-19 pathogenesis.
Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F, Zahmatkeshan M, et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26(1). https://doi.org/10.1186/s12929-019-0563-4.
Suara R, Crowe J. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004;48(3):783–90. https://doi.org/10.1128/aac.48.3.783-790.2004.
Ferrari E, Wright-Minogue J, Fang J, Baroudy B, Lau J, Hong Z. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J Virol. 1999;73(2):1649–54. https://doi.org/10.1128/jvi.73.2.1649-1654.1999.
Bhutta Z, Black R, Brown K, Gardner J, Gore S, Hidayat A, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr. 1999;135(6):689–97. https://doi.org/10.1016/s0022-3476(99)70086-7.
Mehta P, McAuley D, Brown M, Sanchez E, Tattersall R, Manson J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. https://doi.org/10.1016/S0140-6736(20)30628-0.
Cackman I, Kirchner H, Rink L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J Interf Cytokine Res. 1997;17(8):469–72. https://doi.org/10.1089/jir.1997.17.469.
Bauer S, Kapoor A, Rath M, Thomas S. What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19? Cleve Clin J Med. 2020. https://doi.org/10.3949/ccjm.87a.ccc046This article discusses the potential role of supplementation of vitamin C, zinc, and vitamin D for the prevention and treatment of COVID-19. These vitamins and minerals have a role in reducing inflammation and promoting the immune response in the host.
Derbyshire E, Delange J. COVID-19: is there a role for immunonutrition, particularly in the over 65s? BMJ Nutr Prev Health. 2020;3(1):100–5. https://doi.org/10.1136/bmjnph-2020-000071.
Galli F, Reglero G, Bartolini D, Visioli F. Better prepare for the next one. Lifestyle lessons from the COVID-19 pandemic. PharmaNutrition. 2020;12:100193. https://doi.org/10.1016/j.phanu.2020.100193This review identifies factors shown to reduce the burden of disease following the COVID-19 pandemic. The highlighted features include the essential role of confinement and healthy lifestyles and nutrition emphasizing the importance of micronutrients and vitamins as immunoprotective agents.
Rahman M, Idid S. Can Zn be a critical element in COVID-19 treatment? Biol Trace Elem Res. 2020;199:1–9. https://doi.org/10.1007/s12011-020-02194-9.
Prasad A, Beck F, Bao B, Fitzgerald J, Snell D, Steinberg J, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85(3):837–44. https://doi.org/10.1093/ajcn/85.3.837.
Fraker P, King L, Laakko T, Vollmer T. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130(5):1399S–406S. https://doi.org/10.1093/jn/130.5.1399S.
Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Effect of zinc supplementation on cell-mediated immunity and lymphocyte subsets in preschool children. Indian Pediatr. 1997;34:589–97.
Wessells K, Brown K. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11):e50568. https://doi.org/10.1371/journal.pone.0050568.
Shittu MO, Afolami OI. Improving the efficacy of chloroquine and hydroxychloroquine against SARS-CoV-2 may require zinc additives—a better synergy for future COVID-19 clinical trials. Infez Med. 2018;28(2):192–7 This review highlights the potential benefit of using zinc and chloroquine in the fight against COVID-19. Chloroquine is able to induce the uptake of zinc into cells ultimately inhibiting its replication through the inhibition of RNA-dependent RNA polymerase.
Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19? Med Hypotheses. 2020;142:109815. https://doi.org/10.1016/j.mehy.2020.109815.
Moll R, Davis B. Iron, vitamin B 12 and folate. Medicine. 2017;45(4):198–203. https://doi.org/10.1016/j.mpmed.2017.01.007.
Crider K, Yang T, Berry R, Bailey L. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv Nutr. 2012;3(1):21–38. https://doi.org/10.3945/an.111.000992.
Heiser K, McLean P, Davis C, Fogelson B, Gordon H, Jacobson P, et al. Identification of potential treatments for COVID-19 through artificial intelligence-enabled phenomic analysis of human cells infected with SARS-CoV-2 (Preprint). 2020. https://doi.org/10.1101/2020.04.21.054387.
Maggini S, Wintergerst E, Beveridge S, Hornig D. Contribution of selected vitamins and trace elements to immune function. Proc Nutr Soc. 2008;67(OCE1). https://doi.org/10.1017/s0029665108006939.
Carr A, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. https://doi.org/10.3390/nu9111211.
Mousavi S, Bereswill S, Heimesaat M. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. 2019;9(3):73–9. https://doi.org/10.1556/1886.2019.00016.
Erol A. High-dose intravenous vitamin C treatment for COVID-19. 2020. Available on: https://osf.io/p7ex8/?fbclid=IwAR2b67345SBs9r2QfJ23xH_0GEM771Qwww6EPpOSTSpQ7_x2BUu7-5CZEHo. https://doi.org/10.31219/osf.io/p7ex8. Accessed August 18 2020. This review suggests the possible role of high-dose intravenous vitamin C as a treatment option for the early stages of COVID-19 as this has been shown to be effective against sepsis and septic shock.
Bozonet S, Carr A, Pullar J, Vissers M. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold Kiwifruit. Nutrients. 2015;7(4):2574–88. https://doi.org/10.3390/nu7042574.
Parker W, Rhea E, Qu Z, Hecker M, May J. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am J Phys Cell Phys. 2016;311(4):C652–62. https://doi.org/10.1152/ajpcell.00076.2016.
Cheng R. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5:100028. https://doi.org/10.1016/j.medidd.2020.100028This review suggests the use of high dose oral vitamin C for the treatment against COVID-19. Vitamin C’s antioxidant role has been shown to reduce the effect of the cytokine storm observed in patients with SARS-CoV-2 infection.
Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11(4):708. https://doi.org/10.3390/nu11040708.
Fowler A, Truwit J, Hite R, Morris P, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure. JAMA. 2019;322(13):1261–70. https://doi.org/10.1001/jama.2019.11825.
Clinicaltrials.gov. Vitamin C infusion for the treatment of severe 2019-nCoV infected pneumonia' (Identifier: NCT04264533). 2020. Retrieved 18 August 2020, from https://clinicaltrials.gov/ct2/show/NCT04264533?term=NCT04264533&draw=2&rank=1. Accessed August 18 2020.
Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):5. https://doi.org/10.1186/s13613-020-00792-3.
Clinicaltrials.gov. Early infusion of vitamin C for treatment of novel COVID-19 acute lung injury (EVICT-CORONA-ALI) (Identifier: NCT04344184). 2020. Retrieved 18 August 2020, from https://clinicaltrials.gov/ct2/show/NCT04344184?term=NCT04344184&draw=2&rank=1. Accessed August 18 2020.
Thacher T, Clarke B. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. https://doi.org/10.4065/mcp.2010.0567.
Greiller C, Martineau A. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–70. https://doi.org/10.3390/nu7064240.
Beard JA, Bearden A, Striker R. Vitamin D and the antiviral state. J Clin Virol. 2011;50(3):194–200. https://doi.org/10.1016/j.jcv.2010.12.006.
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96. https://doi.org/10.1016/j.coph.2010.04.001.
Kamen D, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88(5):441–50. https://doi.org/10.1007/s00109-010-0590-9.
Arboleda J, Urcuqui-Inchima S. Vitamin D supplementation: a potential approach for coronavirus/COVID-19 therapeutics? Front Immunol. 2020;11. https://doi.org/10.3389/fimmu.2020.01523.
Jakovac H. COVID-19 and vitamin D—Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5):E589. https://doi.org/10.1152/ajpendo.00138.2020This review highlights the therapeutic benefits of vitamin D in modulating viral replication and subsequent viral mediated respiratory inflammation. The researchers emphasise the importance of investigating Vitamin D as a prophylactic and adjuvant therapy in the context of viral infection.
Grant W, Baggerly C, Lahore H. Reply: “Vitamin D supplementation in influenza and COVID-19 infections. Comment on: Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths nutrients 2020, 12(4), 988”. Nutrients. 2020;12(6):1620. https://doi.org/10.20944/preprints202003.0235.v2.
Honardoost M, Ghavideldarestani M, Khamseh ME. Role of vitamin D in pathogenesis and severity of COVID-19 infection. Arch Physiol Biochem. 2020:1–7. https://doi.org/10.1080/13813455.2020.1792505This article highlights the role of vitamin D in the pathogenesis and severity of COVID-19 infection by disrupting the interaction between the virus and target cells but also via enhancing the host's immunological potential.
Gowda P, Sen E. Involvement of bradykinin and folate receptor in calming the SARS-COV2 cytokine storm: lessons learnt from lung cancer Research Square (preprint). https://doi.org/10.21203/rs.3.rs-23656/v.
Martín Giménez V, Inserra F, Tajer C, Mariani J, Ferder L, Reiter R, et al. Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 2020;254:117808. https://doi.org/10.1016/j.lfs.2020.117808.
Carter S, Baranauskas M, Fly A. Considerations for obesity, vitamin D, and physical activity amid the COVID-19 pandemic. Obesity. 2020;28(7):1176–7. https://doi.org/10.1002/oby.22838.
Wacker M, Holick MF. Sunlight and vitamin D. Dermato-Endocrinology. 2013;5(1):51–108. https://doi.org/10.4161/derm.24494.
Rhodes J, Subramanian S, Laird E, Kenny R. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434–7. https://doi.org/10.1111/apt.15777.
Hribar C, Cobbold P, Church F. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain Sci. 2020;10(5):284. https://doi.org/10.3390/brainsci10050284This review highlights the antiviral and immunomodulatory features of vitamin D, which are suggested to play a role in improving Parkinson related pathogenesis. The researchers also discuss how vitamin D may offer antiviral protection from SAR-CoV-2 through immunomodulation.
Panarese A, Shahini E. Letter: Covid-19, and vitamin D. Aliment Pharmacol Ther. 2020;51(10):993–5. https://doi.org/10.1111/apt.15752This article examines the function of ACE2 as the docking host receptor for SARS-CoV-2 and its subsequent expression modulated by vitamin D. This review concludes the potential use of vitamin D as prophylaxis in reducing symptom severity of patients who are experiencing hypovitaminosis D.
Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466. https://doi.org/10.3390/nu12051466.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Public Health Nutrition
Rights and permissions
About this article
Cite this article
Pinnawala, N.U., Thrastardottir, T.O. & Constantinou, C. Keeping a Balance During the Pandemic: a Narrative Review on the Important Role of Micronutrients in Preventing Infection and Reducing Complications of COVID-19. Curr Nutr Rep 10, 200–210 (2021). https://doi.org/10.1007/s13668-021-00356-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-021-00356-2