Abstract
The potential of cultivar mixtures to reduce disease severity and increase yields in cereals across the globe is well established. The effect of cultivar mixtures on the selection for pathogen strains resistant to specific fungicides has, however, not previously been investigated. In this study, the case of the pathogen Zymoseptoria tritici causing Septoria tritici blotch in wheat (Triticum aestivum) and resistance development to azole fungicides by single mutations in CYP51 was explored. Cultivar mixtures composed of a range of resistant and susceptible winter wheat cultivars were grown across a total of seven field trial sites and three growing seasons. The treatments consisted of untreated plots and plots with one, two, or three fungicide applications. From the trials, the economically optimal fungicide input was calculated and the level of fungicide resistance was measured as the frequency of key CYP51 mutations. The study demonstrates for the first time how cultivar mixtures can reduce the selection for fungicide resistance and can reduce the need for fungicide input. Based on four trial sites in two growing seasons, the majority of cultivar mixtures reduced the frequency of a CYP51 mutation compared with the component cultivars in pure stand. The highest significant reduction in mutation frequency by a cultivar mixture was 73%. Conditions with high fungicide input and low disease severity resulted in the most pronounced reductions in mutation frequency by cultivar mixtures. The economical need for using fungicides was also impacted by cultivar mixtures when compared with pure stand. Based on six trial sites across two growing seasons, the majority (67%) of cultivar mixtures had the potential to reduce the number of fungicide applications compared with their pure stand counterparts. These findings could have notable implications for intensive crop production. Within-field diversity can reduce the threat from diseases that have become resistant to fungicides and contribute to creating a more sustainable production where lower chemical inputs can sustain high yields.
Similar content being viewed by others
1 Introduction
One of the major threats to crop production is the development of fungicide resistance. An important case is the wheat (Triticum aestivum) pathogen, Zymoseptoria tritici, which causes Septoria tritici blotch (STB) and is distributed worldwide (Eyal, 1987). STB is the most yield-limiting disease in European wheat production and is widely relying on fungicides for control (Fones and Gurr 2015; Torriani et al. 2015). The control of STB is threatened by the development of fungicide resistance. The efficacy of some of the most used fungicides (demethylation inhibitors, DMIs) has in some cases been reduced from ≈ 80% control to ≈ 40% control over a period of 6 years (Jørgensen et al. 2018). Yearly yield losses from STB can reach 10–30% if no control measures are applied (Jørgensen et al. 2014). There is a need for effective anti-resistance strategies to avoid further development of fungicide resistance resulting in extensive yield losses and to protect new fungicides on the market. The most well-documented anti-resistance strategies are currently lowering the fungicide dose and mixing different fungicide modes of action (van den Bosch et al. 2014; Heick et al. 2017a). As these methods do not fully mitigate fungicide resistance development but rather slow down the development, it is beneficial to develop other anti-resistance strategies. A previously unstudied approach is the utilization of genetic diversity in the host cultivars.
The importance of biodiversity for the function of natural ecosystems is by now well established as well as the role of intensive, uniform cropping systems in the spread of plant diseases (McDonald and Stukenbrock, 2016). In agriculture, there is a growing understanding that knowledge about diversity in natural ecosystems can contribute to more sustainable crop production (Barot et al. 2017; Østergå̊rd et al., 2009). A simple way of introducing more diversity into agro-ecosystems is the use of cultivar mixtures that are known for their ability to reduce fungal diseases in cereal crops (Borg et al. 2018; Finckh et al. 2000; Wolfe 1985). It has been demonstrated by Barek et al. (2019); Cowger and Mundt (2002); Gigot et al. (2013); Kristoffersen et al. (2019); Mille et al. (2006); Vidal et al. (2017) that cultivar mixtures can reduce the severity of STB.
The possible role of cultivar mixtures in the selection for fungicide resistance has not previously been explored. A theoretic possibility of cultivar mixtures interfering with fungicide resistance exists on the basis of three factors. First, cultivar resistance genes and fungicides can be used interchangeably for disease control as they both reduce disease severity by limiting the growth rate of the pathogen (Carolan et al., 2017). Second, different cultivars can select differently for mutations linked to fungicide resistance as observed by Vagndorf et al. (2018), where some CYP51 mutations were significantly more frequent in isolates derived from specific cultivars. Vagndorf et al. (2018) propose a link between pathogen preference for certain cultivars and specific CYP51 mutations under the influence of fungicides. A third theory to support the possibility of mixtures interacting with fungicide is that a mechanism for disease reduction by cultivar mixtures is “disrupted selection.” Disrupted selection limits the epidemic development of a specific fungal strain with an affinity for a specific cultivar due to the spatial distribution of cultivars and thereby reduces the selection towards specific cultivars (Borg et al., 2018). If cultivars and fungicides can be used interchangeably and cultivars have an impact on selection for fungicide resistance, cultivar mixtures could work as an extension of the anti-resistance strategy of mixing modes of action. Unlike the mixing of fungicides, the mixed cultivars will be spatially distributed and can lead to a disrupted selection. As the cultivars can differ in affinity for specific strains carrying fungicide resistance mutations, the disrupted selection could affect the overall selection for fungicide resistance in the field. Additionally, numerous studies showed that cultivar mixtures increase the yield of cereals, particularly when plant diseases were a limiting factor (Borg et al. 2018; Kiær et al. 2009; Reiss and Drinkwater 2018). This produces a theoretical possibility that in a situation with a substantial disease pressure, the yield increase from a cultivar mixture could be greater than the yield increase from a fungicide application. If cultivar mixtures can reduce the need for fungicide application, they can indirectly reduce the development of fungicide resistance by mitigating the selection pressure.
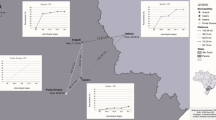
This study was established with cultivar mixtures designed for STB control (Fig. 1) to test if selection to fungicide resistance could be reduced by cultivar mixtures in practice. We propose that cultivar mixtures can reduce the number of fungicide applications. This will have an indirect impact on fungicide resistance by lessening the selection pressure. We further propose that cultivar mixtures have a direct impact on fungicide resistance by disruptive selection. As the pathogen’s selection towards overcoming the resistance genes of a specific cultivar is disrupted when the cultivar is in a mixture, the selection towards fungicide resistance is likewise impaired. The frequency of mutations linked to reduced sensitivity to azole fungicides will therefore be reduced when cultivars are grown in a mixture. CYP51 mutations V136A, V136C, D134G, and S524T were chosen as indicator mutations for the impact of cultivar mixtures on fungicide resistance. These are all among the mutations assumed to have the highest effect on the sensitivity to azole fungicides (Leroux and Walker, 2011). The frequency of V136A in the European Z. tritici population has increased in recent years; the selection of haplotypes harboring this mutation, often in combination with D134G, is a result of the widespread use of the fungicides epoxiconazole and prothioconazole (Heick et al. 2017b; Kildea et al. 2019). S524T has been linked to reduced sensitivity of most azole fungicides and especially prothioconazole (Cools et al., 2011).
2 Materials and methods
2.1 Field trials
2.1.1 Trial conditions 2017–2019
Field trials were conducted at a total of seven locations across Denmark in 2017–2019 (Table 1). These were Flakkebjerg (55° 19′ 35.2″ N, 11° 23′ 16.9 E), Horsens (55° 51′ 31.4″ N, 9° 43′ 04.8″ E), Labing (56° 10′ 11.4″ N, 10° 00′ 28.8″ E), Odense (55° 17′ 48.1″ N, 10° 36′ 20.3″ E), Ringsted (55° 27′ 26.1″ N, 11° 57′ 14.4″ E), Sønderborg (54° 58′ 05.0″ N, 9° 42′ 21.4″ E), and Ultang (55° 12′ 44.6″ N, 9° 39′ 07.6″ E). The growing season of 2017 was characterized by high rainfall and precipitation of 160.5 mm between April and June (measured at trial location Flakkebjerg). In 2018, the growing season was unusually dry and only 77.6 mm of rain fell between April and June resulting in very poor conditions for STB development. The growing season of 2019 had a precipitation of 123.7 mm. In 2017 and 2018, the trials were mainly affected by STB. In 2019, conditions were favorable for both STB and yellow rust (Puccinia striiformis). The cultivar Benchmark was seen to be highly susceptible to yellow rust.
2.1.2 Experiment A: Cultivar mixtures grouped by susceptibility
Field experiment A was conducted to evaluate cultivar mixtures of either susceptible or resistant cultivars under different fungicide programs of varying intensity. The experiment included six pure stand cultivars and two cultivar mixtures. Susceptibility score for the cultivars (0–100) was computed from the previous growing season (2016) in the official cultivar testing (available at sortinfo.dk). One mixture was composed of the three susceptible cultivars: Benchmark (Sejet Plant Breeding, STB score: 12), Torp (Nordic Seed A/S, STB score: 13), and Hereford (Sejet Plant Breeding, STB score: 24). The other cultivar mixture was composed of the three resistant cultivars: Sheriff (Sejet Plant Breeding, STB score: 2.8), Kalmar (Nordic Seed A/S, STB score: 5), and Creator (Sejet Plant Breeding, STB score: 3.4). The susceptibility of Kalmar increased in 2017 and was replaced in the 2018 trial by the cultivar Informer (Breun Seed GmbH Co. KG). The field experiment was carried out at two trial sites: Flakkebjerg and Horsens (Table 1). At trial site Flakkebjerg, 22 m2 plots were sown in a split-plot design with the cultivars grouped. The trial included three replicates of each treatment sown in blocks. The treatments were randomized in two of the blocks; in the third block, treatments were not randomized as the block was used for demonstrating the treatments. At trial site Horsens, 12.5 m2 plots were sown in a split-plot design with three replicates. Different fungicides were applied with active ingredients that covered the three major modes of action: demethylation inhibitors (DMIs), succinate dehydrogenase inhibitors (SDHIs), and quinone outside inhibitors (QoIs) (Table 2). The treatments were designed to cover varying doses and number of treatments with conventional fungicides (Table 2). The treatments were applied using a plot sprayer with flat fan nozzles and a water volume of 150 l/ha. Additional plant protection and fertilization were applied according to standard practice. Early onset of mildew was controlled with a selective mildew fungicide if necessary. The Flakkebjerg trial site was irrigated two times with 25 mm water in 2018 and one time with 20 mm in 2019.
A spraying error at the second fungicide application in 2017 at location Flakkebjerg led to a large number of plots being discarded across treatments and cultivars. For the fungicide resistance test, leaves of untreated, pure stand cultivars were collected from neighboring field trials to replace missing values in the analysis. The trial was discarded from the yield analysis. The entire trial at location Horsens was excluded in 2018 due to drought.
2.1.3 Experiment B: Cultivar mixtures of mixed susceptibility
Experiment B was conducted to evaluate cultivar mixtures of mixed susceptibility under different fungicide programs of varying intensity and included the same cultivars as experiment A except Hereford and Creator. The cultivar mixtures included resistant cultivars (R), medium susceptible cultivars (M), and susceptible cultivars (S).
In 2017, the mixtures were R R S (Kalmar, Sheriff, Torp), R S S (Sheriff, Torp, Benchmark), and R R S S (Kalmar, Sheriff, Torp, Benchmark). In 2018, the mixtures were R M S (Informer, Kalmar, Benchmark), R M S S (1/4 Informer, 1/4 Kalmar, 2/4 Benchmark), MS (Kalmar, Benchmark), and M S S (1/3 Kalmar, 2/3 Benchmark). In 2019, the mixtures were R R (Informer, Sheriff), R R M (Informer, Sheriff, Kalmar), R R M S (Informer, Sheriff, Kalmar, Benchmark), and R R S (Informer, Sheriff, Benchmark).
The field experiment was carried out at five trial sites (Table 1). All trial sites were sown as randomized block designs with four replicates in 12.5 m2 plots. The fungicide treatments were either untreated or conventional treatments with two or three applications (Table 2). In 2019, the treatments were adjusted for two and three applications and treatment with one application was added (Table 2). Additional plant protection and fertilization were applied according to standard practice. The field trials were planned and conducted by SEGES (the Danish Farmers Union).
In 2017, the trial site Odense was discarded due to too high variations in yield, and trial site Ringsted was discarded due to lack of disease. In 2018, trial sites Ringsted, Odense, and Labing were discarded due to drought. In total, 11 trials were used for further analysis.
2.1.4 Yield and treatment cost
To calculate and compare the optimum fungicide input for cultivar mixtures and pure stand cultivars, grain yield, and treatment costs were measured. Grain yield was measured at harvest and adjusted to 15% moisture content. The net yield was calculated as grain yield multiplied by grain price minus the cost of the fungicide application. The grain price was set to 13.3 €/hkg and fungicide prices of 2018 were used. The price per liter product of Viverda was €57.3, Bell €60, Proline EC 250 €64.9, and Prosaro EC250 €46. Viverda was added 1:1 with the adjuvant Ultimate S (BASF A/S) at a price of 5.6 €/L. An application cost of 9.3 € per treatment was included as calculated by the Danish Knowledge Center for Agriculture, SEGES (Pedersen, 2018).
2.2 Test for azole-resistant mutations in CYP51
To test whether cultivar mixtures had an influence on the selection for fungicide resistance, a number of CYP51 mutations were tested for in leaf samples collected from a number of field experiments in 2017 and 2018. Trial site Flakkebjerg (2017 and 2018) and trial site Horsens (2017) were included from field experiment A. Trial site Sønderborg (2017 and 2018) and trial site Ultang (2018) were included from field experiment B. Samples from 2017 were collected from all treatments and pooled across blocks. In 2018, samples were collected from a subset of the treatments, which allowed room for all replicates to be included. In 2018, samples were collected from untreated plots, one treatment (high) and three treatments (high) in experiment A and untreated plots in experiment B.
The samples were collected at GS 75 (BBCH) from the highest leaf level with symptoms. This was L1 (flag leaf) for samples collected in 2017 and L2 (leaf below flag leaf) for samples collected in 2018. Minimum 10 leaves were collected at random per plot. The collected leaves were dried at room temperature and ground to a fine powder with a Geno Grinder® 2010 (Spex® SamplePrep, Stanmore, U.K.) at 1500 rpm in 15–25 min depending on the sample.
Genomic DNA was extracted from the infected leaf material. Samples from the year 2017 were extracted manually using the Qiagen DNeasy®Plant Mini extraction kit (Qiagen GmbH, Hildesheim, Germany) according to the manufacturer’s protocol. Samples from 2018 were extracted through an automated process with the Speadex®maxi plant kit and a KingFisher 96 (Thermo Fisher Scientific, Massachusetts, USA) instrument following the manufacturer’s protocol. Both methods yielded DNA amounts in the same range.
The frequency of the mutations (V136A, V136C, D134G, and S524T) in the CYP51 enzyme linked to azole fungicide resistance was detected using pyrosequencing as described by Sierotzki et al. (2019) and Stammler et al. (2008). All reactions were performed in duplicate and constituted the technical replicates. A qPCR assay was performed for S524T using a Rotor-Gene-Q (Qiagen GmbH, Hildesheim, Germany) and Takyon™ No Rox Probe MasterMix dTTP (Eurogentec, Seraing, Belgium). Data for S524T was only available for 2017.
2.3 Data analysis
Yield and mutation data was fitted with linear mixed models in the form of observation∼cultivar:treatment + (1∣block) + (1∣block:cultivar) for split-plot trials or observation∼cultivar:treatment + (1∣block) for randomized block design using R and the package lme4. As the 2017 fungicide resistance data did not have true replicates, the effects were assessed across treatments, and treatment was then used as a random factor. The effect of the mixture relative to the component cultivars in the pure stand was expressed as a log response ratio. The log response ratio is the natural logarithm of the ratio between the mixture estimate over the average of the pure stand estimates of component cultivars. The delta method and the package car were used to estimate the mean effect size and confidence interval for the mixtures. Model control was carried out through analysis of QQ-plots and residuals plots. None of the data was transformed. Outliers from the fungicide resistance test were removed manually.
The comparisons between pure stand cultivars and mixtures in regard to the number of fungicide treatments were made step-by-step as described in Fig. 2. The initial step excludes trials where there was no need for control with fungicides in the pure stand to avoid false-positive reductions by the mixture.
3 Results and discussion
3.1 Effect of cultivar mixtures on fungicide use
Yield data was available from 11 trials and ranged from 5–11 t/ha. In eight trials, fungicide treatments significantly increased the net yield of the pure stand cultivars. All trials were from 2017 and 2019 when disease pressure was high. Across these trials, a total of 24 mixtures were tested for their ability to reduce the number of treatments compared with the component cultivars as pure stand cultivars (Table 3). In eight cases, the mixture was unable to reduce the number of fungicide applications, and a reduction in treatment number resulted in a significant yield loss. In nine cases, reducing the number of treatments in the mixture did not result in a significant decrease in net yield. These were listed as “possibly” able to reduce treatment number. In seven cases, the estimated net yield was higher for the mixture with a reduced fungicide input compared with the pure stand cultivars. For three of these, the mixture was able to reduce the treatment number from one or two to none. In total, 16 of 24 mixtures (67%) were either able to or possibly able to reduce the number of treatments.
There was a pattern in mixture composition and the ability to reduce the fungicide application number. For the seven cases with “yes” to the question “can the mixture reduce treatment number?” Four of them were mixtures with four components. For the nine cases where the answer was “possibly,” two had four components. For the eight cases where the answer was “no,” only one was a four-component mixture. The majority “yes” cases had high variation in susceptibility and three of four-way mixtures were RRMS mixtures. None of the “yes” cases had 2/3 or more resistant cultivars in the mixture. This was four out of nine (44%) of the “possibly” cases and seven out of eight (88%) for the “no” cases.
Farmers aim for high yields and especially high net yields. If cultivar mixtures can produce equally high net yields as the pure stand cultivars but with less fungicide input, the farmer can justify reducing fungicide treatments. The mixtures did not unequivocally reduce the need for treatment with broad-spectrum fungicides compared with their pure stand counterparts based on the 24 cases in this study. In total, 29% of the mixtures could clearly reduce the number of fungicide treatments necessary. If a non-significant yield loss was accepted when reducing treatment number, the majority (67%) of cultivar mixtures were able to reduce fungicide input. This would then support the hypothesis that cultivar mixtures can indirectly affect the selection for fungicide resistance. However, the majority of mixtures followed the same yield response pattern as the component pure stand cultivars.
The information about the successful cases can be used to indicate what type of cultivar mixtures can be used to reduce the fungicide input. The number of cultivars and the ratio of susceptible to resistant cultivars are commonly found to affect yield and disease severity in a mixture (Borg et al. 2018; Finckh et al. 2000; Mille et al. 2006; Reiss and Drinkwater 2018). A similar pattern was found in the composition of cultivar mixtures in this study on the ability to reduce treatment number. Mixtures with large differences in susceptibility and with more components were more likely to reduce treatment number. An exception was the mixture with only susceptible cultivars. However, this mixture still had very large differences in susceptibility with the inclusion of the older and highly susceptible cultivar, Hereford, that had a severity score that was almost the double of the severity scores for the other cultivars in the mixture.
The net yield is dependent on the treatment cost and the treatment cost is therefore closely linked to the potential of cultivar mixtures to reduce the need for fungicide input. The hypothesis is based on fewer treatments being less expensive than more treatments. The treatment “one treatment (high)” could not be used in this case as it was more expensive than two treatments. The mixture’s ability to reduce treatment number is highly dependent on fungicide efficacy, yield response, and the fungicide price at a given time and place. A part of the fungicide price in Denmark is a pesticide tax—in 2019, the tax for Viverda was 62% of the price and 13% for Proline EC 250 (middeldatabasen.dk). In countries with a different regulation or overall lower prices, a reduced treatment number due to the use of cultivar mixtures might not lead to an increased net yield. Opposite, in areas with lower yield potential, the yield increase from fungicide treatments might in fewer cases be able to pay for the fungicide cost.
3.2 Selection for fungicide resistance
The selection for fungicide resistance varied across trial sites and cultivars. The range for the mutation V136A was 45–80% with the majority above 65%. Four out of five cases with values below 65% were at location Flakkebjerg and two of these were the cultivar Hereford. Location Horsens was predominantly present in the lower to mid-range and location Sønderborg was more present in the mid to high range. More trials from 2017 were present in the lower range and more trials from 2018 were present in the higher range. The D134G mutation in the samples was at the same levels as V136A. V136C varied between 3 and 25%. S524T was only assessed in 2017 and varied between 1 and 45% with the majority below 10%. The level of CyP51 mutations from this study was in line with other Danish investigations (Heick et al., 2017b). The fungicide treatments had an impact on the selection of the different mutations. On average, all treatments increased the frequency of V136A, while fungicide treatments decreased the frequency of V136C and D134G in most cases. The changes were more pronounced in 2017 than in 2018.
The majority of cultivar mixtures were able to reduce the selection for mutations in the CYP51 enzyme linked with reduced sensitivity to azole fungicides (Table 4). For the V136A mutation, 10 of the 15 mixtures reduced selection compared with the components in pure stand. Of these, five significantly reduced the selection. One mixture significantly increased selection for the V136A mutation. For the V136C mutation, 12 of the 15 mixtures reduced selection. Of these, five significantly reduced the selection. One mixture significantly increased the selection. The highest reductions by mixtures were found for V136C with up to 80% reduction of the mutation. For the D134G mutation, seven of the 14 mixtures reduced selection compared with the components in pure stand. Of these, six significantly reduced the selection. Two mixtures significantly increased selection for the D134G mutation. For the S524T mutation, five of the seven mixtures reduced selection compared with the components in pure stand. Of these, one significantly reduced the selection.
The mixtures treated with “three treatments (high)” at Flakkebjerg in 2018 consistently reduced the selection across mutations compared with the component cultivars in pure stand. Three of the six reductions were both above 40% and significant. In the same trial, four out of six in the untreated mixtures significantly reduced selection, but at levels below 20%. At trial location Sønderborg in 2017, the cultivar mixtures exhibited substantial and significant reductions and five out of nine were significant reductions. Trial location Horsens in 2017 had no significant reductions and two significant increases. Mixtures treated with “one treatment (high)” were among the least effective in reducing selection with increases in mutation frequency for both V136A and D134G. Cultivar mixtures did, however, on average decrease selection for the V136C mutation. The results were inconsistent at trial locations Sønderborg and Ultang in 2018.
The results, to a large extent, supported the hypothesis that cultivar mixtures have a direct impact on the selection for mutations linked to fungicide resistance. This analysis did, however, not include enough different mixtures to conclude whether any specific type of mixture performed better than the others. Mixing different fungicide modes of action is an effective way to slow down selection for fungicide resistance (van den Bosch et al. 2014; Dooley et al. 2016; Heick et al. 2017a). Theoretically, different cultivars could act as different modes of action of fungicides by varying in their selection of specific mutations linked to fungicide resistance. Vagndorf et al. (2018) found that the frequency of specific mutations based on samples collected at the same site differed significantly between cultivars. Young et al. (2018) observed differences in the selection of V136A within the same site for cultivars with different susceptibilities to STB. In this study, different cultivars were associated with different frequencies of specific mutations, but this varied highly between locations and years. There was some indication that location and year had an impact on the mixtures’ ability to reduce the selection, as trial location, Flakkebjerg had consistently high reductions, as did Sønderborg in 2017, whereas Horsens in 2017 and Sønderborg and Ultangin 2018 had fewer reductions. The treatment appeared to be a factor to influence the mixture impact on the selection the most. Both cultivar mixtures treated with “three treatments (high)” in 2018 reduced selection to all three mutations compared with their pure stand counterparts. This is arguably the case with the highest selection pressure towards fungicide resistance as well in a year with very low disease pressure and a treatment with a high fungicide input. Cultivar mixtures are often observed to be more effective at reducing the disease when the disease pressure is high. Similarly, cultivar mixtures could be more likely to reduce selection for fungicide resistance when the selection pressure is high. On average, the two mutations with the lowest frequency in the population, V136C and S524T, were the mutations that were reduced the most by cultivar mixtures. This is in contrast to the dynamics for diseases where cultivar mixtures typically have a higher impact on disease severity when the disease pressure is high (Finckh et al. 2000; Reiss and Drinkwater 2018). However, strategies to reduce fungicide resistance development have a greater impact on early stages (van den Bosch et al. 2011; Hobbelen et al. 2014) and cultivar mixtures are thus more likely to reduce amputation that is less established in the population.
One of the main concerns for the use of cultivar mixtures is that they will lead to the development of complex races of the pathogen that can overcome the resistance genes of several cultivars (Finckh et al., 2000). The results from this study could indicate that the pathogen population is unable to adapt to both different cultivars and fungicides at the same time. To avoid the development of complex races, it might be beneficial to change some of the component cultivars of a mixture between growing seasons and vary the fungicide mode of action used. For the latter point, a limited number of available modes of action could restrict this strategy.
4 Conclusion
The research on the mitigation of fungicide resistance in cereals has previously focused on optimizing the management of fungicide input. Meanwhile, the research on cultivar mixtures has been focused on quantifying yield gains and disease reductions. As the role of intensive, uniform cropping systems in the spread of plant diseases as well as the benefits of using concepts from natural ecosystems has become better understood, there is great potential in the use of diversity in the broader fight against plant diseases. Genetic diversity, in the form of cultivar mixtures, has the potential to reduce the need for fungicide input due to their ability to both increase yields and reduce disease severity. As fungicides and resistant cultivars both slow the growth of the disease and as cultivar mixtures can disrupt the selection for host susceptibility in the pathogen population the interactions are likely to have an impact on the selection for fungicide resistance as well.
This study has shown for the first time that cultivar mixtures contribute to a reduced selection of fungicide resistance and have the potential to reduce fungicide input. The results support the hypothesis that cultivar mixtures can disrupt the selection in the pathogen population towards both fungicides and host resistance genes. Cultivar mixtures then have the potential to prolong the longevity of cultivars and fungicides. As the results of this study to a large extent confirm the existence of both a direct and an indirect impact of cultivar mixtures on fungicide resistance, it would be useful to include cultivar mixtures in strategies aiming at anti-resistance and integrated pest management. By combining existing anti-resistance strategies and cultivar mixtures, there is a potential to enhance disease control and reduce the risk of resistance development against both resistant cultivars and fungicides. To improve the efficacy of cultivar mixtures, more research into the design of mixtures that have the highest impact on fungicide need and selection would be needed.
The results of this study highlight how diversity in cropping systems can lead to beneficial interactions on more levels. This is could extend to more than interactions between cultivars and fungicides. To better understand the advantages of diversifying, it can be necessary to think outside a familiar scope and look broader at what factors are impacted by increased diversity.
References
Barek SBM, Karisto P, Fakhfakh M, Kouki H, Mikaberidze A, Yahyaoui A (2019) Improved control of Septoria tritici blotch in durum wheat using cultivar mixtures. bioRxiv, 664078
Barot S, Allard V, Cantarel A, Enjalbert J, Gauffreteau A, Goldringer I, Lata J-C, Le Roux X, Niboyet A, Porcher E (2017) Designing mixtures of varieties for multifunctional agriculture with the help of ecology. A review. Agron. Sustain. Dev. 37(2):13
Borg, J, Kiær, LP, Lecarpentier, C, Goldringer, I, Gauffreteau, A, Saint-Jean, S, Barot, S, and Enjalbert, J, (2018). Unfolding the potential of wheat cultivar mixtures: a meta-analysis perspective and identification of knowledge gaps. F Crop Res, 221(September 2017):298–313
Carolan K, Helps J, van den Berg F, Bain R, Paveley N, van den Bosch F (2017) Extending the durability of cultivar resistance by limiting epidemic growth rates. Proc. R. Soc. B 284:20170828 The Royal Society
Cools HJ, Mullins JG, Fraaije BA, Parker JE, Kelly DE, Lucas JA, Kelly SL (2011) Impact of recently emerged sterol 14α-demethylase (CYP51) variants of Mycosphaerella graminicola on azole fungicide sensitivity. Appl Environ Microbiol 77(11):3830–3837
Cowger C, Mundt CC (2002) Effects of wheat cultivar mixtures on epidemic progression of Septoria tritici blotch and pathogenicity of Mycosphaerella graminicola. Phytopathology 92(6):617–623
Dooley H, Shaw MW, Spink J, Kildea S (2016) The effect of succinate dehydrogenase inhibitor/azole mixtures on selection of Zymoseptoria tritici isolates with reduced sensitivity. Pest Manag Sci 72(6):1150–1159
Eyal Z (1987) The Septoria diseases of wheat: concepts and methods of disease management. CIMMYT
Finckh MR, Gacek ES, Goyeau H, Lannou C, Merz U, Mundt CC, Munk L, Nadziak J, Newton AC, de Vallavieille-Pope C, Wolfe MS (2000) Cereal variety and species mixtures in practice, with emphasis on disease resistance. Agronomie 20(7):813–837
Fones H, Gurr S (2015) The impact of Septoria tritici blotch disease on wheat: an EU perspective. Fungal Genet Biol 79:3–7
Gigot C, Saint-Jean S, Huber L, Maumené C, Leconte M, Kerhornou B, de Vallavieille-Pope C (2013) Protective effects of a wheat cultivar mixture against splash-dispersed Septoria tritici blotch epidemics. Plant Pathol 62(5):1011–1019
Heick TM, Justesen AF, Jørgensen LN (2017a) Anti-resistance strategies for fungicides against wheat pathogen Zymoseptoria tritici with focus on DMI fungicides. Crop Prot 99:108–117
Heick TM, Justesen AF, Jørgensen LN (2017b) Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic-Baltic region - a status. Eur J Plant Pathol 149(3):669–682
Hobbelen PH, Paveley ND, Van Den Bosch F (2014) The emergence of resistance to fungicides. PLoS One 9(3):1–14
Jørgensen LN, Hovmøller MS, Hansen JG, Lassen P, Clark B, Bayles R, Rodemann B, Flath K, Jahn M, Goral T et al (2014) IPM strategies and their dilemmas including an introduction to www. eurowheat. org. J. Integr. Agric. 13(2):265–281
Jørgensen LN, Nielsen BJ, Mathiassen SK, Jensen PK, Kristjansen HS, Heick TM, Vagndorf N, Hartvig P, Sørensen S (2018) Applied crop protection 2018. Technical report. Aarhus University
Kiær LP, Skovgaard IM, Østergå̊rd, H. (2009) Grain yield increase in cereal variety mixtures: a meta-analysis of field trials. F. Crop. Res. 114(3):361–373
Kildea S, Marten-Heick T, Grant J, Mehenni-Ciz J, Dooley H (2019) A combination of target-site alterations, overexpression and enhanced efflux activity contribute to reduced azole sensitivity present in the Irish Zymoseptoria tritici population. Eur J Plant Pathol.:1–12
Kristoffersen R, Jørgensen LN, Eriksen LB, Nielsen GC, Kiær LP (2019) Control of Septoria tritici blotch by winter wheat cultivar mixtures: meta-analysis of 19 years of cultivar trials. bioRxiv
Leroux P, Walker A (2011) Multiple mechanisms account for resistance to sterol 14a-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag Sci 67(1):44–59
McDonald BA, Stukenbrock EH (2016) Rapid emergence of pathogens in agro-ecosystems: global threats to agricultural sustainability and food security. Phil. Trans. R. Soc. B 371(1709):20160026
Mille B, Fraj MB, Monod H, de Vallavieille-Pope C (2006) Assessing four-way mixtures of winter wheat cultivars from the performances of their two-way and individual components. Eur J Plant Pathol 114(2):163–173
Østergå̊rd H, Finckh MR, Fontaine L, Goldringer I, Hoad SP, Kristensen K, Lammerts van Bueren ET, Mascher F, Munk L, Wolfe MS (2009) Time for a shift in crop production: embracing complexity through diversity at all levels. J Sci Food Agric 89(9):1439–1445
Pedersen JB (2018) Sorter, priser, midler og udviklingsstadier. In: Pedersen JB (ed) Overs. over landsforsøgene 2018. SEGES Landbrug og Fødevarer F.m.b.A, pp 371–387
Reiss ER, Drinkwater LE (2018) Cultivar mixtures: a meta-analysis of the effect of intraspecific diversity on crop yield. Ecol Appl 28(1):62–77
Sierotzki H Mehl A Stammler G (2019) Molecular detection methods for fungicide resistance. In: Stevenson KL, McGrath MT, Wyenandt CA (eds) Fungic. Resist. North. Am., second edn. American Phytopathological Society, St. Paul, Minnesota 55121, U.S.A., pp 175–193
Stammler G, Carstensen M, Koch A, Semar M, Strobel D, Schlehuber S (2008) Frequency of different CYP51-haplotypes of Mycosphaerella graminicola and their impact on epoxiconazole-sensitivity and -field efficacy. Crop Prot 27(11):1448–1456
Torriani SF, Melichar JP, Mills C, Pain N, Sierotzki H, Courbot M (2015) Zymoseptoria tritici: a major threat to wheat production, integrated approaches to control. Fungal Genet Biol 79:8–12
Vagndorf N, Heick TM, Justesen AF, Andersen JR, Jahoor A, Jørgensen LN, Orabi J (2018) Population structure and frequency differences of CYP51 mutations in Zymoseptoria tritici populations in the Nordic and Baltic regions. Eur. J. Plant Pathol.:1–15
van den Bosch F, Paveley N, Shaw M, Hobbelen P, Oliver R (2011) The dose rate debate: does the risk of fungicide resistance increase or decrease with dose? Plant Pathol 60(4):597–606
van den Bosch F, Oliver R, van den Berg F, Paveley N (2014) Governing principles can guide fungicide-resistance management tactics. Annu Rev Phytopathol 52:175–195
Vidal T Boixel AL Durand B de Vallavieille-Pope, C, Huber, L, and Saint-Jean, S, (2017). Reduction of fungal disease spread in cultivar mixtures: impact of canopy architecture on rain-splash dispersal and on crop microclimate. Agric For Meteorol, 246(June):154–161
Wolfe MS (1985) The current status and prospects of multiline cultivars and variety mixtures for disease resistance. Annu Rev Phytopathol 23(1):251–273
Young C Boor T Paveley N Fraaije B Van Den Bosch F (2018). Consequences of intensive fungicide use or integrated disease management for fungicide resistance and sustainable control. (588):1–65
Acknowledgments
The authors would like to thank Gerd Stammler and Birgit Wieja at BASF for assistance with the pyrosequencing.
Funding
The field trials in experiment B received funding from Promilleafgiftsfonden for Landbrug.
Author information
Authors and Affiliations
Contributions
Rose Kristoffersen and Lise Nistrup Jørgensen designed experiment A and Lars Bonde Eriksen and Ghita Cordsen Nielsen designed experiment B. The azole resistance test was designed by Thies Marten Heick. Sampling and leaf processing were carried out by Rose Kristoffersen and Gudrun Maria Müller. Data was analyzed by Rose Kristoffersen and the manuscript was written by Rose Kristoffersen and Gudrun Maria Müller in consultation with Lise Nistrup Jørgensen, Thies Marten Heick, Lars Bonde Eriksen, and Ghita Cordsen Nielsen.
Corresponding author
Ethics declarations
Conflict of interest
The author, Rose Kristoffersen, participated in a 1-month internship at the fungicide resistance laboratory at BASF (Limburgerhof, Germany) to analyze the 2018 samples. BASF has not been involved in the design of the experiment or the interpretation of the results. The remaining authors declare that they have no potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kristoffersen, R., Heick, T.M., Müller, G.M. et al. The potential of cultivar mixtures to reduce fungicide input and mitigate fungicide resistance development. Agron. Sustain. Dev. 40, 36 (2020). https://doi.org/10.1007/s13593-020-00639-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-020-00639-y