Abstract
Merkel cell carcinoma (MCC) is an aggressive skin cancer, with a propensity for early metastasis. Therefore, early diagnosis and the identification of novel targets become fundamental. The enzyme nicotinamide N-methyltransferase (NNMT) catalyzes the reaction of N-methylation of nicotinamide and other analogous compounds. Although NNMT overexpression was reported in many malignancies, the significance of its dysregulation in cancer cell phenotype was partly clarified. Several works demonstrated that NNMT promotes cancer cell proliferation, migration, and chemoresistance. In this study, we investigated the possible involvement of this enzyme in MCC. Preliminary immunohistochemical analyses were performed to evaluate NNMT expression in MCC tissue specimens. To explore the enzyme function in tumor cell metabolism, MCC cell lines have been transfected with plasmids encoding for short hairpin RNAs (shRNAs) targeting NNMT mRNA. Preliminary immunohistochemical analyses showed elevated NNMT expression in MCC tissue specimens. The effect of enzyme downregulation on cell proliferation, migration, and chemosensitivity was then evaluated through MTT, trypan blue, and wound healing assays. Data obtained clearly demonstrated that NNMT knockdown is associated with a decrease of cell proliferation, viability, and migration, as well as with enhanced sensitivity to treatment with chemotherapeutic drugs. Taken together, these results suggest that NNMT could represent an interesting MCC biomarker and a promising target for targeted anti-cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine tumor belonging to the non-melanoma skin cancers. Incidence rates of MCC range from 0.1 to 2.5 cases per 100,000 individuals although in the last decades, in different countries, an increase incidence per year has been reported. This tumor usually arises as a fast-growing pink–red dome-shaped nodule, mostly occurring on sun-exposed areas [1]. The name MCC has been historically used due to the structural and morphological characteristics that these tumors cells share with Merkel cells, although recent studies suggested different origins of MCC cells [2].
MCC most commonly develops on the sun-exposed skin of the head and neck (50%), in upper limbs or shoulders (24%), lower limb or hips (15%), trunk (11%) and more rarely in other districts. Risk factors can be viral infection, DNA damage and mutation, as the result of ultraviolet exposure, old age, male sex, and individuals with immunosuppression [2]. Almost 95% patients are fair skinned and MCC is known to be rare in dark-skin individuals. MCC of the head and neck district is very aggressive since it is characterized by a high frequency of recurrence and it is prone to develop early metastases. Indeed, MCC displays a significantly higher 5-year recurrence rate compared to other aggressive skin cancers like melanoma or squamous cell carcinoma [3].
The initial approach for the management of MCC depends on the pathology of the primary cancer and the presence of metastasis. Surgical excision is the primary treatment, with wider margin excisions in case of presence of baseline risk factors. Non-surgical treatment of MCC includes radiotherapy, immunotherapy, and chemotherapy. MCC displays a marked radiosensitivity, thus radiotherapy is effective in controlling the malignancy though systemic relapses are frequent, making the radiotherapy recommended only for patients that are not surgical candidates. Recently, the use of checkpoint immunotherapy has demonstrated to be beneficial for MCC patients, although more finalized to control the disease than reaching a curative aim [4]. MCC is recognized as a chemosensitive malignancy displaying high response rates; thus, chemotherapy is used for the primary therapy of advanced MCC or as an adjuvant treatment. Nonetheless, the duration of response to chemotherapy is poor, with patients suffering from significant toxicity to the therapy [5]. Therefore, it is of outmost importance the identification of new diagnostic and prognostic markers, as well as biomolecules that could be used as target for precision medicine.
In this work, we focused on nicotinamide N-methyltransferase (NNMT), a phase II metabolism enzyme which has found to be overexpressed in several malignancies, contributing to tumor progression [6,7,8,9,10,11,12].
The aim of the present work was to explore the potential involvement of this enzyme in MCC. Immunohistochemistry was performed on a few selected formalin-fixed and paraffin-embedded (FFPE) MCC tissue samples, to evaluate NNMT expression levels. To further investigate the role played by the enzyme in tumor cell metabolism, MCC-13 and MCC-26 Merkel carcinoma cell lines have been transfected with vectors encoding short hairpin RNAs targeting NNMT mRNA, and the efficiency of enzyme knockdown has been assessed by real-time PCR and western blot analysis. Subsequently, we analyzed the impact of NNMT downregulation on MCC cell lines in terms of proliferation, cell viability, and invasiveness. Finally, cell viability of NNMT downregulating cells and controls was analyzed upon treatment with cisplatin, to evaluate the potential involvement of the enzyme in sensitivity of MCC cells to chemotherapy.
Materials and methods
Immunohistochemistry
A retrospective analysis was conducted in accordance with the principles of the Declaration of Helsinki on a total of 11 FFPE MCC specimens, collected from patients undergoing biopsy or excisional surgery between 2018 and 2020. Tissue samples were obtained from the archives of the Section of Pathology, Department of Biomedical Sciences and Public Health, Polytechnic University of Marche.
5 µm sections from FFPE blocks were processed as previously described [13]. Analyses were independently performed by two investigators blinded to the patient group (E.S. and G.G.), using a Nikon Eclipse E600 light microscope equipped with a Nikon DS-Vi1 digital camera (Nikon Instruments, Europe BV, Kingston, Surrey, England). Stained cells were counted in at least ten fields per sample (field area: 0.07 mm2, magnification: × 400) and quantified as a percentage of the total counted cells. Agreement between observers was always > 95%. Discrepancies were resolved by simultaneous reexamination of the slides, using a double-headed microscope.
Cell lines and culture conditions
MCC-13 [14] and MCC-26 [15] cells were cultured in Dulbecco’s modified Eagle medium (DMEM) High Glucose media added with 10% fetal bovine serum (FBS) (Euroclone, Milan, Italy) and 50 μg/mL gentamicin, at 37℃ in a humified 5% CO2 incubator.
NNMT shRNA-mediated gene silencing
To achieve NNMT knockdown, MCC-13 and MCC-26 cells were seeded in 24-well plates (7 × 104 cells/well) and grown in complete medium until approximately 80% confluence at the moment of transfection, that was performed using FuGENE HD Transfection Reagent (Promega, Madison, WI, USA). The day after, cells were transfected with vectors (0.5 µg/well) encoding short hairpin RNAs (shRNAs) targeting different regions of NNMT transcript (pLKO.1–164, target nucleotide sequence 5ʹ-ACCCTCGGGATTACCTAGAAA-3ʹ, pLKO.1–330, target nucleotide sequence 5ʹ-CCTCTCTGCTTGTGAATCCTT-3ʹ, and pLKO.1–448, target nucleotide sequence 5ʹ- GTGACCTATGTGTGTGATCTT-3ʹ). Control cells were transfected with empty vector (pLKO.1-puro) or treated with transfection reagent only (mock). 48 h following transfection, cellular clones stably downregulating NNMT started to be selected in complete medium containing 1 μg/mL puromycin, to select, for each sample, those cellular clones containing vector conferring resistance to puromycin. For this reason, medium was changed every 2 days, until the complete death of mock cells, while puromycin-resistant cells were maintained in selection medium. NNMT knockdown efficiency was analyzed by real-time PCR and western blot analysis.
Real-time quantitative PCR
RNA was extracted from cancer cell pellets (1 × 106) using the SV Total RNA Isolation System (Promega, Madison, WI, USA). 2 μg of RNA was reverse transcribed with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) and 1 µL of the cDNA mixture was used for real-time PCR, as previously described [16]. Fold changes in relative gene expression were calculated by 2−ΔΔCt, where ΔCt = Ct (NNMT) − Ct (housekeeping gene) and Δ(ΔCt) = ΔCt (NNMT silencing vector) − ΔCt (empty vector).
Western blot analysis
Western blot analysis was set up to evaluate NNMT protein levels, as reported elsewhere [16]. Blot was probed with rabbit polyclonal antibody against NNMT (Sigma-Aldrich; cat. No. SAB1100302-200UL) (1:1,000 dilution) or with mouse monoclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology; cat. No. sc-47724) (1:250), for 1 h, followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Sigma-Aldrich; cat. No. A0545-1ML) (1:150,000 dilution) or with HRP-conjugated goat anti-mouse IgG (Bio-rad; cat. No. 1706516) (1:5000) for 1 h at room temperature, respectively.
Cell viability and proliferation assays
The assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was used to evaluate cell viability, measuring the capability of mitochondrial dehydrogenase enzymes of living cells to convert tetrazolium salt MTT to formazan. The MTT test was executed in accordance with a previous study [16]. Proliferation of NNMT-silenced cells was estimated by trypan blue exclusion assay. MCC-13 and MCC-26 cells were seeded on 6-well plates at a density of 1 × 105 cells/well in serum-free medium. The day after, representing the 0 h time point, cells were detached using 500 μL trypsin and centrifuged at 300×g for 3 min. Cell pellet was resuspended in 500 μL complete medium, trypan blue was added, and cells were counted using Burker’s chamber. The same procedure was repeated at different time points (24, 48, and 72 h). The number of viable cells was indicated as percentage of the control (number of viable counted cells of each sample at 0 h, corresponding to 100%) and presented as mean values ± standard deviation. Each experiment, in triplicate, was repeated 3 times.
Monolayer wound healing assay
To evaluate cell migration ability, transfected cells were seeded into 6-well plates (3 × 105 cells/well) until 100% confluency. A 200 μL sterile pipette tip was used to obtain the wound on cells monolayers, then cell debris were removed by washing cell monolayers 3 times and the medium was replaced with DMEM High Glucose media containing 0.5% FBS. Monolayers were photographed at 0, 2, 4, 8, 24 h after scratching. Each experiment, in triplicate, was repeated 3 times.
Chemotherapeutic treatment
Concerning the evaluation of the effect induced by NNMT silencing on cell sensitivity to chemotherapy, MCC-26 cells, both those downregulating the enzyme and controls, were treated with cisplatin at different concentrations (0.1 and 1 μg/mL), and the sensitivity to drug was evaluated through MTT assay in accordance with what was previously described [16]. Briefly, cells were seeded in 96-well plates (5 × 103 cells/well). The day after seeding, MCC-26 cells were treated with cisplatin. MTT assay was used to estimate the cell proliferation at different time points (24, 48, and 72 h) after starting treatment with drug.
Statistical analysis
GraphPad Prism software version 8.00 for Windows (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Kruskal–Wallis test was used to evaluate differences between groups. A p value < 0.05 was considered as statistically significant.
Results
NNMT expression in MCC tissue samples
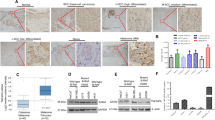
The results obtained by immunohistochemical evaluation evidenced a strong NNMT cytoplasmic expression in more than 90% of the tumor cells in the analyzed fields, while the nuclei showed no immunostaining (Fig. 1).
Efficiency of NNMT silencing in MCC cell lines
Real-time PCR analyses showed that NNMT-silenced MCC-13 cells showed a significant (p < 0.05) enzyme downregulation (0.43 ± 0.039 for pLKO.1–330 and 0.75 ± 0.067 for pLKO.1–448) compared to control cells (1.00 ± 0.078 pLKO.1-puro) (Fig. 2A). In MCC-26 cell line, treatment with pLKO.1–164 (0.24 ± 0.036), pLKO.1–330 (0.69 ± 0.052), and pLKO.1–448 (0.31 ± 0.029) led to a significant reduction of enzyme expression with respect to reference sample (1.00 ± 0.085; pLKO.1-puro) (Fig. 2B). Western blot analysis and densitometry of immunoreactive bands confirmed a decreased NNMT expression in MCC-13 cells transfected with pLKO.1–330 (0.36 ± 0.038) and pLKO.1–448 (0.48 ± 0.058) compared with those treated with empty vector (1.00 ± 0.089; pLKO.1-puro) (Fig. 2C and E), as well as in MCC-26 cells transfected with pLKO.1–164 (0.21 ± 0.032), pLKO.1–330 (0.38 ± 0,042), and pLKO.1–448, (0.30 ± 0.047) with respect to control cells (1.00 ± 0.076; pLKO.1-puro) (Fig. 2D and F).
Evaluation of NNMT silencing in MCC cell lines. MCC-13 (A, C, E) and MCC-26 (B, D, F) cells were transfected with plasmids coding shRNAs targeting NNMT (pLKO.1–164, pLKO.1–330 and pLKO.1–448) and with empty vector as control (pLKO.1-puro). Enzyme expression was then evaluated at mRNA and protein level, by real-time PCR (A, B) and western blot (C, D), followed by densitometry (E, F). Human recombinant his-tagged NNMT and human recombinant his-tagged GAPDH were used as protein standards (29.6 kDa and 36 kDa molecular mass, respectively). Each experiment, in triplicate, was repeated 3 times. Values are expressed as mean ± standard deviation (*p < 0.05)
Effect of NNMT downregulation on proliferation, viability, and migration of MCC cells
In MCC-13 cells downregulating NNMT (pLKO.1–330 and pLKO.1–448), data obtained clearly showed a significant (p < 0.05) reduction in cell growth after 24, 48, and 72 h compared to control cells (pLKO.1-puro) (Fig. 3A). In MCC-26 cells, enzyme downregulation resulted in significant reduction of cell growth after 24, 48, and 72 h in cells treated with pLKO.1–164, and only after 72 h in cells treated with pLKO.1–330 and pLKO.1–448 (Fig. 3C). Results collected from the trypan blue exclusion assay revealed that NNMT silencing led to a significant (p < 0.05) decrease of cell proliferation in both MCC-13 (Fig. 3B) and MCC-26 (Fig. 3D) cells harboring shRNA plasmids against NNMT with respect to that of reference samples (pLKO.1-puro) at 48 and 72 h time points.
In vitro effect of NNMT silencing on cell viability and proliferation. Cell viability was measured by MTT (A, C) and trypan blue exclusion (B, D) assays, in both MCC-13 (A, B) and MCC-26 (C, D) cell lines. Each experiment, in triplicate, was repeated 3 times. Values are expressed as mean ± standard deviation (*p < 0.05)
Migration capability of MCC-13 and MCC-26 cells was monitored by monolayer wound healing assay. For each sample, data were reported as percentage of wound recovery with respect to 0 h. Compared with control cells, the migration rate of both cells lines significantly (p < 0.05) decreased after NNMT silencing at 4, 8, and 24 h time points for MCC-13 cells (Fig. 4A and C) and at 8 and 24 h time points for MCC-26 cells (Fig. 4B and D).
In vitro effect of NNMT silencing on cell migration. MCC cells were subjected to wound healing assay to evaluate their migration potential. MCC-13 cells transfected with plasmids pLKO.1–330, pLKO.1–448, or pLKO.1-puro (A) and MCC-26 cells transfected with plasmids pLKO.1–164, or pLKO.1-puro (B) were photographed immediately after scratch (0 h) and at different time points, ranging between 2 and 24 h. Migration ability was evaluated by measuring percentage of wound recovery compared with 0 h, as reported in bar diagrams (C or MCC-13 and D for MCC-26). Each experiment, in triplicate, was repeated 3 times. Values are expressed as mean ± standard deviation (*p < 0.05)
Effect of NNMT knockdown on MCC cells sensitivity to chemotherapeutic treatment
Several studies reported in literature clearly demonstrated an NNMT involvement in resistance to chemotherapy. Therefore, MCC-26 cells downregulating NNMT, as well as controls, were treated with cisplatin at different concentration values and then cell viability was assessed using MTT assay. Interestingly, upon treatment with cisplatin at 0.1 and 1 μM, a significantly (p < 0.05) higher decrease in cell viability was shown in NNMT downregulating cells compared with control cells at 72 h time point (Fig. 5).
Impact of NNMT knockdown on chemosensitivity of MCC-26 cells. The effect of cisplatin treatment at 0.1 µg/mL (A) and 1 µg/mL (B) concentrations on viability of MCC-26 cells was evaluated by MTT assay. Measurements were performed at 0, 24, 48, and 72 h time points. Each experiment, in triplicate, was repeated 3 times. All values are expressed as mean ± standard deviation (*p < 0.05)
Discussion
MCC is a rare and extremely aggressive cutaneous malignancy, presenting the highest case-by-case mortality rate among all skin tumors [1]. The clinical presentation of MCC is varied and nonspecific, usually presenting as a rapidly growing solitary cutaneous or subcutaneous erythematous or violaceous nodule [17]. Besides rapid local growth, cancer progression is so fast, with metastasis to the lymph nodes and distal organs. Despite advances in the understanding of the carcinogenesis of MCC, the molecular mechanisms underlying this disease are far to be fully elucidated. Patients are often elderly and the metastatic potential of the tumor is high, so MCC prognosis is poor [18,19,20]. An early diagnosis should be a primary goal and the development of targeted therapies is urgently required.
In the present study, we focused on NNMT enzyme, catalyzing the N-methylation of nicotinamide, pyridines, and other analogs compounds, thus playing an important activity in the transformation of many xenobiotics [21, 22].
Preliminary immunohistochemical analyses, performed in a few selected FFPE MCC tissue samples, showed high NNMT expression. Subsequently, the influence of shRNA-mediated NNMT gene silencing on viability, migration, and proliferation of MCC cell lines was evaluated. Upon assessment of the efficiency of NNMT knockdown by real-time PCR and western blot assay, the effect on cell growth, viability, and migration was analyzed by MTT, trypan blue, and wound healing assays, respectively. Interestingly, our data showed that efficient downregulation of NNMT resulted in significantly reduced cell proliferation and viability, as well as migration and invasiveness. To speculate the relation between enzyme expression and resistance to chemotherapeutic drugs, cell viability of MCC cells downregulating NNMT and controls was evaluated under treatment with increasing concentrations of cisplatin. Interestingly, preliminary data showed that enzyme knockdown significantly enhanced decrease of cell viability following administration with chemotherapeutic drug, suggesting that targeting NNMT could represent a promising strategy to increase the efficacy of chemotherapy.
In our previous works, we reported an enhanced expression of NNMT in numerous cancers, such as renal cell carcinoma (ccRCC) [23], oral squamous cell carcinoma (OSCC) [11], bladder urothelial carcinoma [9], non-small cell lung cancer (NSCLC) [24], and skin malignancies [8, 25].
Although different kind of cancers have been correlated with NNMT dysregulation, its role in cancer cell metabolism remains partly undiscovered. As found in multiple types of human malignancies, NNMT seems to promote both tumorigenicity and proliferation of tumor cells. Many studies have been carried out to elucidate the function of the NNMT in tumorigenesis and cancer cell metabolism, as well as to clarify the significance of its overexpression in different malignancies [26,27,28,29,30,31,32,33,34,35].
In human bladder cancer cell lines, the enzyme silencing led to a significant decrease in cell migration, highlighting the crucial role of the NNMT in tumor metastasis and invasion [30]. Analyses carried out on ccRCC cells treated with shRNAs against NNMT mRNA showed that the enzyme can promote cellular invasiveness both in vitro and in vivo, with the activation of the PI3K/Akt/SP1/MMP-2 pathway [31]. Furthermore, NNMT knockdown in the pancreatic cancer cell line PANC-1 was significantly correlated with reduction of cell proliferation and migration, as well as invasive capacity, suggesting the enzyme involvement in cell proliferation and metastatic potential of tumor cells [32]. In vitro and in vivo experiments of NNMT silencing in Bcap-37 and MDA-MB-231 human breast cancer cell lines showing high enzyme levels led to apoptosis induction and significantly reduced cell growth and tumorigenicity. Converse results were obtained after NNMT upregulation in MCF-7 and SK-BR-3 breast cancer cell lines that lacked constitutive NNMT expression [27]. Equally, NNMT upregulation had been induced in SW480 colorectal cancer cells lines which lack endogenous NNMT expression, whereas NNMT silencing has been performed in HT-29 cells with high constitutive enzyme expression, showing that NNMT enhances cell proliferation and colony formation, inhibits apoptotic pathway, promotes cell cycle, and increases ATP levels [28]. In NSCLC H1993 cells, which displayed high endogenous levels of the enzyme, NNMT knockdown significantly suppressed colony formation capability [36]. In line with these findings, in the PC3 prostate cancer cells, induction of NNMT overexpression promoted cell proliferation, invasive ability, and migration capability, by increasing mRNA levels of histone deacetylase SIRT1 ability [37]. Similarly, treatment of EC9706 and TE1 esophageal squamous cell carcinoma cells with shRNAs targeted to NNMT mRNA significantly suppressed cell migration and viability, promoted apoptosis and cell cycle, inhibited EMT via the Wnt/β-catenin pathway [38]. Our previous studies demonstrated that NNMT silencing in human OSCC and NSCLC cell lines significantly inhibited cell growth and tumorigenicity [39, 40]. Conversely, the induction of NNMT overexpression in the HSC-2 OSCC cell line was significantly associated with enhanced cell proliferation [41], suggesting that the enzyme could play a fundamental role in the tumorigenic capability and proliferation of tumor cells, and the possibility that NNMT could represent a therapeutic target for the management of several solid malignancies.
Currently, the specific mechanism by which NNMT can influence tumorigenesis and tumor progression has not been completely clarified. NNMT could modify the SAM:SAH ratio affecting the methylation state of cancer cells, but also store N1-methylnicotinamide into tumor cells, thus fine-tuning the methylation state of cancer cells [42]. Moreover, NNMT activity is involved in the regulation of the homeostasis of nicotinamide, a specific precursor of the NAD + . Therefore, the catalytic activity of the NNMT can control the amount of nicotinamide inside the cell available for the energy metabolism, thus influencing and modulating multiple pathways linked to both death and viability [43]. These pathways involve NAD-dependent PARPs and SIRT1 enzymes, that are important for the correct DNA repair which prevents the tumor transformation [43, 44].
Different studies reported in literature clearly demonstrated an NNMT involvement in resistance to chemotherapy. Enzyme downregulation decreased resistance to 5-fluorouracil (5-FU) in human colorectal cell line HT-29, while NNMT overexpression induced the opposite effect in the colorectal cell line SW480. The capability of the enzyme to increase chemosensitivity was found to be mediated by its reaction product N1-methylnicotinamide, which reduces the production of reactive oxygen species induced by 5-FU treatment [28]. Furthermore, a recent work showed that NNMT knockdown increases 5-FU sensitivity of esophageal squamous cell carcinoma cell line TE1 via suppressing Warburg effect, while overexpression of the enzyme in EC1 and Eca109 cells increased the 5-FU sensitivity, thus suggesting that NNMT can represent a potential therapeutic target to enhance the therapeutic activity of 5-FU [45]. Results obtained in our recent study clearly demonstrated that NNMT knockdown led to a significant reduction of cell proliferation and migration of melanoma cell lines. Moreover, the enzyme downregulation in melanoma cells was associated with an increased sensitivity to treatment with dacarbazine, suggesting that NNMT could be involved in mechanisms promoting melanoma cell resistance to chemotherapy [16].
The present study is in line with the available literature ascribing to the NNMT, an important role in promoting cancer cell proliferation, invasiveness, and chemoresistance. Nonetheless, we are aware of the limitations of our study. The immunohistochemical analyses performed in this study involved a limited cohort of patients since MCC is an extremely rare malignancy; thus, further investigations should be focused on evaluating the immunohistochemical expression of NNMT in a larger cohort of patients. Moreover, it would be necessary to validate our interesting in vitro findings in an in vivo model, to confirm the possibility of exploiting a targeted anti-cancer therapy based on the use of specific inhibitors of the enzyme in the management of MCC. In this context, studies providing a mechanistic insight would also be necessary.
Conclusion
This is the first study that highlights the involvement of NNMT in MCC, supporting the hypothesis that the enzyme could represent an interesting biomarker for treatment and detection of this kind of tumor. Indeed, based on the results obtained from cellular experiments, NNMT seems to be involved in tumor cell proliferation, migration, and resistance to chemotherapy, thus representing a potential target for counteracting tumor growth. Although further experiments are necessary to understand the specific mechanisms by which NNMT could participate to MCC tumorigenesis, our results seem to indicate that this enzyme may provide an interesting molecular target for cancer therapy.
Data availability
The data that support the findings of this study are available from the corresponding authors, DS and RC, upon reasonable request.
Abbreviations
- ccRCC:
-
Clear cell renal cell carcinoma
- DMEM:
-
Dulbecco’s modified Eagle medium
- FBS:
-
Fetal bovine serum
- FFPE:
-
Formalin-fixed and paraffin-embedded
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HRP:
-
Horseradish peroxidase
- MCC:
-
Merkel cell carcinoma
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NNMT:
-
Nicotinamide N-methyltransferase
- NSCLC:
-
Non-small cell lung cancer
- OSCC:
-
Oral squamous cell carcinoma
- shRNAs:
-
Short hairpin RNAs
References
Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol. 2016; 23(11): 3564–3571.
Sunshine JC, Jahchan NS, Sage J, ChoiJ. Are there Multiple Cells of Origin of Merkel Cell Carcinoma? Oncogene. 2018; 37(11): 1409–1416.
McEvoy AM, LachancenK, Hippe DS, Cahill K, Moshiri Y, Lewiset CW, et al. Recurrence and Mortality Risk of Merkel Cell Carcinoma by Cancer Stage and Time from Diagnosis. JAMA Dermatol. 2022; 158(4): 382–389.
Angeles CV, Sabel MS. Immunotherapy for Merkel cell carcinoma. J Surg Oncol. 2021;123(3):775–81.
Duarte-Bateman D, Shen A, Bullock T, Sadeghi P, Escandón JM, Dedkova E, et al. Best practices in surgical and nonsurgical managementof head and neck Merkel cell carcinoma: An update. Mol Carcinog. 2023;62(1):101–12.
Pozzi V, Campagna R, Sartini D, Emanuelli M. Nicotinamide N-Methyltransferase as Promising Tool for Management of Gastrointestinal Neoplasms. Biomolecules. 2022;12(9):1173.
Wang W, Yang C, Wang T, Deng H. Complex roles of nicotinamide N-methyltransferase in cancer progression. Cell Death Dis. 2022;13(3):267.
Sartini D, Molinelli E, Pozzi V, Campagna R, Salvolini E, Rubini C, et al. Immunohistochemical expression of nicotinamide N-methyltransferase in lymph node metastases from cutaneous malignant melanoma. Hum Cell. 2023;36(1):480–2.
Campagna R, Pozzi V, Spinelli G, Sartini D, Milanese G, Galosi AB, et al. The Utility of Nicotinamide N-Methyltransferase as a Potential Biomarker to Predict the Oncological Outcomes for Urological Cancers: An Update. Biomolecules. 2021;11(8):1214.
Li XY, Pi YN, Chen Y, Zhu Q, Xia BR. Nicotinamide N-Methyltransferase: A Promising Biomarker and Target for Human Cancer Therapy. Front Oncol. 2022;12:894744.
Togni L, Mascitti M, Sartini D, Campagna R, Pozzi V, Salvolini E, et al. Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules. 2021;11(11):1594.
Ganzetti G, Sartini D, Campanati A, Rubini C, Molinelli E, Brisigotti V, et al. Nicotinamide N-methyltransferase: potential involvement in cutaneous malignant melanoma. Melanoma Res. 2018;28(2):82–8.
Pompei V, Salvolini E, Rubini C, Lucarini G, Molinelli E, Brisigotti V, et al. Nicotinamide N-methyltransferase in nonmelanoma skin cancers. Eur J Clin Invest. 2019;49:e13175.
Leonard JH, Dash P, Holland P, Kearsley KH, Bell JR. Characterisation of four Merkel cell carcinoma adherent cell lines. Int J Cancer. 1995;60(1):100–7.
Van Gele M, Leonard JH, Van Roy N, Van Limbergen H, Van Belle S, Cocquyt V, et al. Combined karyotyping, CGH and M-FISH analysis allows detailed characterization of unidentified chromosomal rearrangements in Merkel cell carcinoma. Int J Cancer. 2002;101(2):137–45.
Campagna R, Salvolini E, Pompei V, Pozzi V, Salvucci A, Molinelli E, et al. Nicotinamide N-methyltransferase gene silencing enhances chemosensitivity of melanoma cell lines. Pigment Cell Melanoma Res. 2021;34(6):1039–48.
Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78(3):433–42.
Walsh NM, Cerroni L. Merkel cell carcinoma: A review. J Cutan Pathol. 2021;48(3):411–21.
Zwijnenburg EM, Lubeek SFK, Werner JEM, et al. Merkel cell carcinoma: New trends. Cancers (Basel). 2021;13(7):1614.
Patel P, Hussain K. Merkel cell carcinoma. Clin Exp Dermatol. 2021;46(5):814–9.
Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994; 269: 14835–14840.
Peng Y, Sartini D, Pozzi V, Wilk D, Emanuelli M, Yee VC. Structural basis of substrate recognition in human nicotinamide N-methyltransferase. Biochemistry. 2011;50:7800–8.
Campagna R, Cecati M, Pozzi V, Fumarola S, Pompei V, Milanese G, et al. Involvement of transforming growth factor beta 1 in the transcriptional regulation of nicotinamide N-methyltransferase in clear cell renal cell carcinoma. Cell Mol Biol (Noisy-le-grand). 2018; 64(7): 51–55.
Sartini D, Morganti S, Guidi E, Rubini C, Zizzi A, Giuliante R, et al. Nicotinamide N-methyltransferase in Non-small Cell Lung Cancer: Promising Results for Targeted Anti-cancer Therapy. Cell Biochem Biophys. 2013;67:865–73.
Campagna R, Pozzi V, Sartini D, Salvolini E, Brisigotti V, Molinelli E, et al. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers (Basel). 2021;13(19):4943.
Zhang J, Wang Y, Li G, Yu H, Xie X. Down-regulation of nicotinamide N-methyltransferase induces apoptosis in human breast cancer cells via the mitochondria-mediated pathway. PLoS ONE. 2014;9:e89202.
Xie X, Yu H, Wang Y, Zhou Y, Li G, Ruan Z, et al. Nicotinamide N-methyltransferase enhances the capacity of tumorigenesis associated with the promotion of cell cycle progression in human colorectal cancer cells. Arch Biochem Biophys. 2014;564:52–66.
Xie X, Liu H, Wang Y, Zhou Y, Yu H, Li G, et al. Nicotinamide N-methyltransferase enhances resistance to 5-fluorouracil in colorectal cancer cells through inhibition of the ASK1-p38 MAPK pathway. Oncotarget. 2016;7:45837–48.
Win KT, Lee SW, Huang HY, Lin LC, Lin CY, Hsing CH, et al. NicotinamideN-methyltransferase overexpression is associated with Akt phosphorylation and indicates worse prognosis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34:3923–31.
Wu Y, Siadaty MS, Berens ME, Hampton GM, Theodorescu D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene. 2008;27:6679–89.
Tang SW, Yang TC, Lin WC, Lin WC, Chang WH, Wang CC, Lai MK, et al. Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis. 2011;32:138–45.
Yu T, Wang YT, Chen P, Li YH, Chen YX, Zenget H, al. Effects of nicotinamide N-methyltransferase on PANC-1 cells proliferation, metastatic potential and survival under metabolic stress. Cell Physiol Biochem. 2015; 35: 710–721.
Chen C, Wang X, Huang X, Yong H, Shen J, Tang Q, et al. Nicotinamide N-methyltransferase: a potential biomarker for worse prognosis in gastric carcinoma. Am J Cancer Res. 2016;6:649–63.
Parsons RB, Aravindan S, Kadampeswaran A, Evans EA, Sandhu KK, Levy ER, et al. The expression of nicotinamide N-methyltransferase increases ATP synthesis and protects SH-SY5Y neuroblastoma cells against the toxicity of complex I inhibitors. Biochem J. 2011;436:145–55.
Zhou W, Gui M, Zhu M, Long Z, Huang L, Zhou J, et al. Nicotinamide Nmethyltransferase is overexpressed in prostate cancer and correlates with prolonged progression-free and overall survival times. Oncol Lett. 2014;8:1175–80.
Bach D, H Kim D, Bae SY, Kim WK, Hong JY, Lee HJ et al. Targeting nicotinamide N-methyltransferase and miR-449a in EGFR-TKI-resistant non-small-cell lung cancer cells. Molecular Therapy -Nucleic Acids. 2018; 11: 455–467.
You Z, Liu Y, Liu X. Nicotinamide N-methyltransferase enhances the progression of prostate cancer by stabilizing sirtuin. Oncol Lett. 2018;15:9195–201.
Cui Y, Zhang L, Wang W, Ma S, Liu H, Zang X, et al. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/β-catenin pathway. Mol Cell Biochem. 2018;460:93–103.
Sartini D, Seta R, Pozzi V, Morganti S, Rubini C, Zizzi A, et al. Role of nicotinamide N-methyltransferase in non-small cell lung cancer: In vitro effect of shRNA-mediated gene silencing on tumourigenicity. Biol Chem. 2015;396(3):225–34.
Pozzi V, Sartini D, Morganti S, Giuliante R, Di Ruscio G, Santarelli A, et al. RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells. PLoS ONE. 2013;8: e71272.
Seta R, Mascitti M, Campagna R, Sartini D, Fumarola S, Santarelli A, et al. Overexpression of nicotinamide N-methyltransferase in HSC-2 OSCC cell line: Effect on apoptosis and cell proliferation. Clin Oral Invest. 2019;23:829–38.
Ulanovskaya OA, Zuhl AM, Cravatt BF. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat Chem Biol. 2013;9:300–6.
Pissios P. Nicotinamide N-methyltransferase: More than a vitaminmB3 clearance enzyme. Trends Endocrinol Metab. 2017;28:340–53.
Mateuszuk Ł, Campagna R, Kutryb-Zając B, Kuś K, Słominska EM, Smolenski RT, et al. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem Pharmacol. 2020;178:114019.
Cui Y, Yang D, Wang W, Zhang L, Liu H, Ma S, et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol Carcinog. 2020;59:940–54.
Acknowledgements
We greatly acknowledge Prof. Baki Akgül, Institute of Virology, University of Cologne, Cologne, Germany for providing Merkel cell carcinoma cell lines.
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement. This study was not supported by any specific funding.
Author information
Authors and Affiliations
Contributions
Valentina Pozzi: methodology, formal analysis, investigation, writing original draft preparation. Elisa Molinelli: formal analysis, data curation. Roberto Campagna: investigation, data curation, writing, review and editing. Emma Nicol Serritelli: methodology, investigation. Monia Cecati: validation, visualization. Edoardo De Simoni: software. Davide Sartini: formal analysis, data curation, writing, review and editing. Gaia Goteri: validation; resources. Nathaniel I. Martin: data curation. Matthijs J. van Haren: data curation. Eleonora Salvolini: data curation, resources. Oriana Simonetti: visualization. Annamaria Offidani: conceptualization, resources. Monica Emanuelli: conceptualization, resources, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors state no conflict of interest.
Ethical approval
The local Institutional Review Board deemed the study exempt from review. According to the rules provided by our Regional Ethical Committee, no approval is needed, since our study is classified as retrospective.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pozzi, V., Molinelli, E., Campagna, R. et al. Knockdown of nicotinamide N-methyltransferase suppresses proliferation, migration, and chemoresistance of Merkel cell carcinoma cells in vitro. Human Cell 37, 729–738 (2024). https://doi.org/10.1007/s13577-024-01047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-024-01047-0