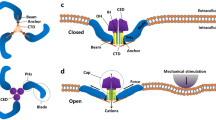
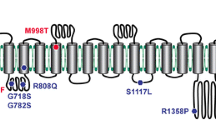
Abstract
Piezo ion channel is a mechanosensitive protein on the cell membrane, which contains Piezo1 and Piezo2. Piezo channels are activated by mechanical forces, including stretch, matrix stiffness, static pressure, and shear stress. Piezo channels transmit mechanical signals that cause different downstream responses in the differentiation process, including integrin signaling pathway, ERK1/2 MAPK signaling pathway, Notch signaling, and WNT signaling pathway. In the fate of stem cell differentiation, scientists found differences in Piezo channel expression and found that Piezo channel expression is related to developmental diseases. Here, we briefly review the structure and function of Piezo channels and the relationship between Piezo and mechanical signals, discussing the current understanding of the role of Piezo channels in stem cell fate and associated molecules and developmental diseases. Ultimately, we believe this review will help identify the association between Piezo channels and stem cell fate.


Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62(3):329–37. https://doi.org/10.18388/abp.2015_1023.
Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. https://doi.org/10.1038/ncomms10366.
Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014;111(45):16148–53. https://doi.org/10.1073/pnas.1409802111.
Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7(1):17696. https://doi.org/10.1038/s41598-017-18089-0.
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA. 2014;111(28):10347–52. https://doi.org/10.1073/pnas.1409233111.
Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. Overview of therapeutic ultrasound applications and safety considerations. J Ultras Med. 2012;31(4):623–34. https://doi.org/10.7863/jum.2012.31.4.623.
Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279–82. https://doi.org/10.1038/nature13701.
Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527(7576):64–9. https://doi.org/10.1038/nature15247.
Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126(12):4527–36. https://doi.org/10.1172/jci87343.
Bai WY, Wang L, Ying ZM, Hu B, Xu L, Zhang GQ, et al. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone. 2020;133:115247. https://doi.org/10.1016/j.bone.2020.115247.
Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife. 2020. https://doi.org/10.7554/eLife.52779.
Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife. 2019. https://doi.org/10.7554/eLife.49631.
Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife. 2019. https://doi.org/10.7554/eLife.47454.
Schrenk-Siemens K, Wende H, Prato V, Song K, Rostock C, Loewer A, et al. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat Neurosci. 2015;18(1):10–6. https://doi.org/10.1038/nn.3894.
Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019;573(7772):130–4. https://doi.org/10.1038/s41586-019-1484-9.
Morley LC, Beech DJ, Walker JJ, Simpson NAB. Emerging concepts of shear stress in placental development and function. Mol Hum Reprod. 2019;25(6):329–39. https://doi.org/10.1093/molehr/gaz018.
Hennes A, Held K, Boretto M, De Clercq K, Van den Eynde C, Vanhie A, et al. Functional expression of the mechanosensitive PIEZO1 channel in primary endometrial epithelial cells and endometrial organoids. Sci Rep. 2019;9(1):1779. https://doi.org/10.1038/s41598-018-38376-8.
Del Mármol JI, Touhara KK, Croft G, MacKinnon R. Piezo1 forms a slowly-inactivating mechanosensory channel in mouse embryonic stem cells. Elife. 2018. https://doi.org/10.7554/eLife.33149.
Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573(7773):225–9. https://doi.org/10.1038/s41586-019-1505-8.
Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019;573(7773):230–4. https://doi.org/10.1038/s41586-019-1499-2.
Wang Y, Xiao B. The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction. J Physiol. 2018;596(6):969–78. https://doi.org/10.1113/jp274404.
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. https://doi.org/10.1126/science.1193270.
Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554(7693):487–92. https://doi.org/10.1038/nature25743.
Zhao Q, Zhou H, Li X, Xiao B. The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019;286(13):2461–70. https://doi.org/10.1111/febs.14711.
Bavi N, Cortes DM, Cox CD, Rohde PR, Liu W, Deitmer JW, et al. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nat Commun. 2016;7:11984. https://doi.org/10.1038/ncomms11984.
Fang XZ, Zhou T, Xu JQ, Wang YX, Sun MM, He YJ, et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021;11(1):13. https://doi.org/10.1186/s13578-020-00522-z.
Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554(7693):481–6. https://doi.org/10.1038/nature25453.
Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, et al. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat Commun. 2015;6:7223. https://doi.org/10.1038/ncomms8223.
Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, et al. Ion permeation and mechanotransduction mechanisms of mechanosensitive Piezo channels. Neuron. 2016;89(6):1248–63. https://doi.org/10.1016/j.neuron.2016.01.046.
Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, et al. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016;17(7):1739–46. https://doi.org/10.1016/j.celrep.2016.10.033.
Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87(6):1162–79. https://doi.org/10.1016/j.neuron.2015.08.032.
Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015. https://doi.org/10.7554/eLife.12088.
Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–81. https://doi.org/10.1038/nature10812.
Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin). 2012;6(4):282–9. https://doi.org/10.4161/chan.21064.
Sun Y, Yong KM, Villa-Diaz LG, Zhang X, Chen W, Philson R, et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater. 2014;13(6):599–604. https://doi.org/10.1038/nmat3945.
Przybyla L, Lakins JN, Weaver VM. Tissue mechanics orchestrate wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell. 2016;19(4):462–75. https://doi.org/10.1016/j.stem.2016.06.018.
Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE. 2010;5(12):e15655. https://doi.org/10.1371/journal.pone.0015655.
Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. Elife. 2020;9:e52779. https://doi.org/10.7554/eLife.52779.
Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife. 2019;8:e49631. https://doi.org/10.7554/eLife.49631.
Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife. 2019;8:47454. https://doi.org/10.7554/eLife.47454.
Nourse JL, Pathak MM. How cells channel their stress: interplay between Piezo1 and the cytoskeleton. Semin Cell Dev Biol. 2017;71:3–12. https://doi.org/10.1016/j.semcdb.2017.06.018.
Botello-Smith WM, Jiang W, Zhang H, Ozkan AD, Lin YC, Pham CN, et al. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun. 2019;10(1):4503. https://doi.org/10.1038/s41467-019-12501-1.
Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015. https://doi.org/10.7554/eLife.07369.
Lacroix JJ, Botello-Smith WM, Luo Y. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat Commun. 2018;9(1):2029. https://doi.org/10.1038/s41467-018-04405-3.
Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q, et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun. 2018;9(1):1300. https://doi.org/10.1038/s41467-018-03570-9.
Xiao B. Levering mechanically activated piezo channels for potential pharmacological intervention. Annu Rev Pharmacol Toxicol. 2020;60:195–218. https://doi.org/10.1146/annurev-pharmtox-010919-023703.
Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol. 2018;175(10):1744–59. https://doi.org/10.1111/bph.14188.
Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89(4):1341–78. https://doi.org/10.1152/physrev.00032.2008.
Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun. 2017;8(1):1797. https://doi.org/10.1038/s41467-017-01712-z.
Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295–300. https://doi.org/10.1021/bi200770q.
Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J. 2017;112(1):31–45. https://doi.org/10.1016/j.bpj.2016.11.013.
Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279(1):19–25. https://doi.org/10.1074/jbc.M311201200.
Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol. 2016;594(3):641–55. https://doi.org/10.1113/jp271714.
Preyer S, Pfister M, Hemmert W. Mechanical stimulation of isolated outer hair cells as a test system. Inhibition of transduction by streptomycin treatment. HNO. 1993;41(10):471–4.
Guo T, Ren P, Li X, Luo T, Gong Y, Hao S, et al. Neural injuries induced by hydrostatic pressure associated with mass effect after intracerebral hemorrhage. Sci Rep. 2018;8(1):9195. https://doi.org/10.1038/s41598-018-27275-7.
Miyazaki A, Sugimoto A, Yoshizaki K, Kawarabayashi K, Iwata K, Kurogoushi R, et al. Coordination of WNT signaling and ciliogenesis during odontogenesis by piezo type mechanosensitive ion channel component 1. Sci Rep. 2019;9(1):14762. https://doi.org/10.1038/s41598-019-51381-9.
Ru J, Guo L, Ji Y, Niu Y. Hydrostatic pressure induces osteogenic differentiation of adipose-derived mesenchymal stem cells through increasing lncRNA-PAGBC. Aging. 2020;12(13):13477–87. https://doi.org/10.18632/aging.103448.
Karamesinis K, Spyropoulou A, Dalagiorgou G, Katsianou MA, Nokhbehsaim M, Memmert S, et al. Continuous hydrostatic pressure induces differentiation phenomena in chondrocytes mediated by changes in polycystins, SOX9, and RUNX2. J Orofac Orthop Fortschritte der Kieferorthopadie. 2017;78(1):21–31. https://doi.org/10.1007/s00056-016-0061-1.
Maki K, Nava MM, Villeneuve C, Chang M, Furukawa KS, Ushida T, et al. Hydrostatic pressure prevents chondrocyte differentiation through heterochromatin remodeling. J Cell Sci. 2021. https://doi.org/10.1242/jcs.247643.
Montagne K, Onuma Y, Ito Y, Aiki Y, Furukawa KS, Ushida T. High hydrostatic pressure induces pro-osteoarthritic changes in cartilage precursor cells: a transcriptome analysis. PLoS ONE. 2017;12(8):e0183226. https://doi.org/10.1371/journal.pone.0183226.
Jin Y, Li J, Wang Y, Ye R, Feng X, Jing Z, et al. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 2015;85(1):87–94. https://doi.org/10.2319/123113-955.1.
Stavenschi E, Corrigan MA, Johnson GP, Riffault M, Hoey DA. Physiological cyclic hydrostatic pressure induces osteogenic lineage commitment of human bone marrow stem cells: a systematic study. Stem Cell Res Ther. 2018;9(1):276. https://doi.org/10.1186/s13287-018-1025-8.
Bratengeier C, Liszka A, Hoffman J, Bakker AD, Fahlgren A. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J. 2020;34(3):3755–72. https://doi.org/10.1096/fj.201901458R.
He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555(7694):103–6. https://doi.org/10.1038/nature25744.
Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118–21. https://doi.org/10.1038/nature21407.
He J, Fang B, Shan S, Xie Y, Wang C, Zhang Y, et al. Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1. Cell Death Dis. 2021;12(3):226. https://doi.org/10.1038/s41419-021-03481-6.
Gaub BM, Müller DJ. Mechanical stimulation of piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. 2017;17(3):2064–72. https://doi.org/10.1021/acs.nanolett.7b00177.
Sun Y, Liu J, Xu Z, Lin X, Zhang X, Li L, et al. Matrix stiffness regulates myocardial differentiation of human umbilical cord mesenchymal stem cells. Aging (Albany). 2020;13(2):2231–50. https://doi.org/10.18632/aging.202244.
Sun M, Chi G, Li P, Lv S, Xu J, Xu Z, et al. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int J Med Sci. 2018;15(3):257–68. https://doi.org/10.7150/ijms.21620.
Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F, et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/pnas.2001611118.
Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33. https://doi.org/10.1038/nrm2593.
Aglialoro F, Hofsink N, Hofman M, Brandhorst N, van den Akker E. Inside out integrin activation mediated by PIEZO1 signaling in erythroblasts. Front Physiol. 2020;11:958. https://doi.org/10.3389/fphys.2020.00958.
Michael M, Parsons M. New perspectives on integrin-dependent adhesions. Curr Opin Cell Biol. 2020;63:31–7. https://doi.org/10.1016/j.ceb.2019.12.008.
Müller P, Langenbach A, Kaminski A, Rychly J. Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PLoS ONE. 2013;8(7):e71283. https://doi.org/10.1371/journal.pone.0071283.
Kopf J, Petersen A, Duda GN, Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 2012;10:37. https://doi.org/10.1186/1741-7007-10-37.
Lai CF, Cheng SL. Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Min Res. 2005;20(2):330–40. https://doi.org/10.1359/jbmr.041013.
Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2005;33(5):438–46. https://doi.org/10.1165/rcmb.2005-0103OC.
Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, et al. Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation. 2007;115(23):2957–68. https://doi.org/10.1161/circulationaha.106.670026.
Chang SF, Chang CA, Lee DY, Lee PL, Yeh YM, Yeh CR, et al. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc Natl Acad Sci USA. 2008;105(10):3927–32. https://doi.org/10.1073/pnas.0712353105.
Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci USA. 2015;112(38):11783–8. https://doi.org/10.1073/pnas.1507309112.
Guo Z, Ohlstein B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science. 2015. https://doi.org/10.1126/science.aab0988.
Kasahara A, Cipolat S, Chen Y, Dorn GW 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science (New York). 2013;342(6159):734–7. https://doi.org/10.1126/science.1241359.
Caolo V, Debant M, Endesh N, Futers TS, Lichtenstein L, Bartoli F, et al. Shear stress activates ADAM10 sheddase to regulate Notch1 via the Piezo1 force sensor in endothelial cells. Elife. 2020. https://doi.org/10.7554/eLife.50684.
Duchemin AL, Vignes H, Vermot J. Mechanically activated piezo channels modulate outflow tract valve development through the Yap1 and Klf2-Notch signaling axis. Elife. 2019. https://doi.org/10.7554/eLife.44706.
Wang L, Wang X, Ji N, Li HM, Cai SX. Mechanisms of the mechanically activated ion channel Piezo1 protein in mediating osteogenic differentiation of periodontal ligament stem cells via the Notch signaling pathway. Hua Xi Kou Qiang Yi Xue Za Zhi Huaxi Kouqiang Yixue Zazhi West China J Stomatol. 2020;38(6):628–36. https://doi.org/10.7518/hxkq.2020.06.004.
Chen P, Zhang G, Jiang S, Ning Y, Deng B, Pan X, et al. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calc. 2021;97:102431. https://doi.org/10.1016/j.ceca.2021.102431.
Miyazaki T, Kanatani N, Rokutanda S, Yoshida C, Toyosawa S, Nakamura R, et al. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch Histol Cytol. 2008;71(2):131–46. https://doi.org/10.1679/aohc.71.131.
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–40. https://doi.org/10.1074/jbc.M500608200.
Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112(3):750–5. https://doi.org/10.1002/jcb.22994.
Mousawi F, Peng H, Li J, Ponnambalam S, Roger S, Zhao H, et al. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells. 2020;38(3):410–21. https://doi.org/10.1002/stem.3114.
Jiang LH, Mousawi F, Yang X, Roger S. ATP-induced Ca(2+)-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci CMLS. 2017;74(20):3697–710. https://doi.org/10.1007/s00018-017-2545-6.
Riddle RC, Taylor AF, Rogers JR, Donahue HJ. ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. J Bone Min Res. 2007;22(4):589–600. https://doi.org/10.1359/jbmr.070113.
Zippel N, Limbach CA, Ratajski N, Urban C, Luparello C, Pansky A, et al. Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Develop. 2012;21(6):884–900. https://doi.org/10.1089/scd.2010.0576.
Ferrari D, Gulinelli S, Salvestrini V, Lucchetti G, Zini R, Manfredini R, et al. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exp Hematol. 2011;39(3):360–74. https://doi.org/10.1016/j.exphem.2010.12.001.
Peng H, Hao Y, Mousawi F, Roger S, Li J, Sim JA, et al. Purinergic and store-operated Ca(2+) signaling mechanisms in mesenchymal stem cells and their roles in ATP-induced stimulation of cell migration. Stem cells (Dayton). 2016;34(8):2102–14. https://doi.org/10.1002/stem.2370.
Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, et al. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376(6543):737–45. https://doi.org/10.1038/376737a0.
Mortadza SS, Sim JA, Stacey M, Jiang LH. Signalling mechanisms mediating Zn(2+)-induced TRPM2 channel activation and cell death in microglial cells. Sci Rep. 2017;7:45032. https://doi.org/10.1038/srep45032.
Gao Q, Cooper PR, Walmsley AD, Scheven BA. Role of Piezo channels in ultrasound-stimulated dental stem cells. J Endodont. 2017;43(7):1130–6. https://doi.org/10.1016/j.joen.2017.02.022.
Urner S, Kelly-Goss M, Peirce SM, Lammert E. Mechanotransduction in blood and lymphatic vascular development and disease. Adv Pharmacol (San Diego). 2018;81:155–208. https://doi.org/10.1016/bs.apha.2017.08.009.
Yamashiro Y, Thang BQ, Ramirez K, Shin SJ, Kohata T, Ohata S, et al. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc Natl Acad Sci USA. 2020;117(18):9896–905. https://doi.org/10.1073/pnas.1919702117.
Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450(7167):285–8. https://doi.org/10.1038/nature06254.
Zhao Z, Zlokovic BV. Endothelial tip cell finds its way with Piezo1. Neuron. 2020;108(1):5–7. https://doi.org/10.1016/j.neuron.2020.09.011.
Liu TT, Du XF, Zhang BB, Zi HX, Yan Y, Yin JA, et al. Piezo1-mediated Ca(2+) activities regulate brain vascular pathfinding during development. Neuron. 2020;108(1):180-92.e5. https://doi.org/10.1016/j.neuron.2020.07.025.
Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. https://doi.org/10.1038/ncomms2899.
Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–15. https://doi.org/10.1182/blood-2012-04-422253.
Kang H, Hong Z, Zhong M, Klomp J, Bayless KJ, Mehta D, et al. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am J Physiol Cell Physiol. 2019;316(1):C92-c103. https://doi.org/10.1152/ajpcell.00346.2018.
Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13(6):1161–71. https://doi.org/10.1016/j.celrep.2015.09.072.
Etem E, Ceylan GG, Özaydın S, Ceylan C, Özercan I, Kuloğlu T. The increased expression of Piezo1 and Piezo2 ion channels in human and mouse bladder carcinoma. Adv Clin Exp Med. 2018;27(8):1025–31. https://doi.org/10.17219/acem/71080.
Albarran-Juarez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215(10):2655–72. https://doi.org/10.1084/jem.20180483.
Lhomme A, Gilbert G, Pele T, Deweirdt J, Henrion D, Baudrimont I, et al. Stretch-activated Piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. Am J Respir Cell Mol Biol. 2019;60(6):650–8. https://doi.org/10.1165/rcmb.2018-0197OC.
Arishe OO, Ebeigbe AB, Webb RC. Mechanotransduction and uterine blood flow in preeclampsia: the role of mechanosensing Piezo 1 ion channels. Am J Hypertens. 2020;33(1):1–9. https://doi.org/10.1093/ajh/hpz158.
Planas-Paz L, Strilić B, Goedecke A, Breier G, Fässler R, Lammert E. Mechanoinduction of lymph vessel expansion. EMBO J. 2012;31(4):788–804. https://doi.org/10.1038/emboj.2011.456.
Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22(2):430–45. https://doi.org/10.1016/j.devcel.2011.12.020.
Choi D, Park E, Jung E, Cha B, Lee S, Yu J, et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. 2019. https://doi.org/10.1172/jci.insight.125068.
Nonomura K, Lukacs V, Sweet DT, Goddard LM, Kanie A, Whitwam T, et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci USA. 2018;115(50):12817–22. https://doi.org/10.1073/pnas.1817070115.
Fotiou E, Martin-Almedina S, Simpson MA, Lin S, Gordon K, Brice G, et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat Commun. 2015;6:8085. https://doi.org/10.1038/ncomms9085.
Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun. 2015;6:8329. https://doi.org/10.1038/ncomms9329.
Tallapragada NP, Cambra HM, Wald T, Keough Jalbert S, Abraham DM, Klein OD, et al. Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids. Cell Stem Cell. 2021;28(9):1516-32.e14. https://doi.org/10.1016/j.stem.2021.04.002.
Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, et al. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci USA. 2016;113(11):3036–41. https://doi.org/10.1073/pnas.1516036113.
Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun. 2013;4:1682. https://doi.org/10.1038/ncomms2673.
Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci. 2015;18(12):1756–62. https://doi.org/10.1038/nn.4162.
Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aat9897.
Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aat9892.
DelleVedove A, Storbeck M, Heller R, Hölker I, Hebbar M, Shukla A, et al. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am J Hum Genet. 2016;99(5):1206–16. https://doi.org/10.1016/j.ajhg.2016.09.019.
Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, et al. The role of PIEZO2 in human mechanosensation. New Engl J Med. 2016;375(14):1355–64. https://doi.org/10.1056/NEJMoa1602812.
Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541(7636):176–81. https://doi.org/10.1038/nature20793.
Okubo M, Fujita A, Saito Y, Komaki H, Ishiyama A, Takeshita E, et al. A family of distal arthrogryposis type 5 due to a novel PIEZO2 mutation. Am J Med Genet A. 2015;167a(5):1100–6. https://doi.org/10.1002/ajmg.a.36881.
McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HI, et al. Mutations in PIEZO2 cause Gordon syndrome, Marden–Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet. 2014;94(5):734–44. https://doi.org/10.1016/j.ajhg.2014.03.015.
Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc Natl Acad Sci USA. 2013;110(12):4667–72. https://doi.org/10.1073/pnas.1221400110.
Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362(6413):464–7. https://doi.org/10.1126/science.aau6324.
Sun Y, Xu Z, Wang M, Lv S, Wu H, Chi G, et al. Soft matrix combined with BMPR inhibition regulates neurogenic differentiation of human umbilical cord mesenchymal stem cells. Front Bioeng Biotechnol. 2020;8:791. https://doi.org/10.3389/fbioe.2020.00791.
Blumenthal NR, Hermanson O, Heimrich B, Shastri VP. Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels. Proc Natl Acad Sci USA. 2014;111(45):16124–9. https://doi.org/10.1073/pnas.1412740111.
Acknowledgements
This study was funded by Science and Technology Projects of the Education Department of Jilin Province (Grant No. JJKH20211154KJ). The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 31201052) and the Project of the Science and Technology Department of Jilin Province (Grant No. 20210509003RQ). The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Author information
Authors and Affiliations
Contributions
XQ, ZD, MW, and YF participated in the writing. XQ, ZD, and MW designed and prepared the figures and tables. YF was in charge of proofreading manuscripts. LL and LB designed and polished the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, X., Deng, Z., Wang, M. et al. Piezo protein determines stem cell fate by transmitting mechanical signals. Human Cell 36, 540–553 (2023). https://doi.org/10.1007/s13577-022-00853-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00853-8