Abstract
The ability of cells to migrate to the destined tissues or lesions is crucial for physiological processes from tissue morphogenesis, homeostasis and immune responses, and also for stem cell-based regenerative medicines. Cytosolic Ca2+ is a primary second messenger in the control and regulation of a wide range of cell functions including cell migration. Extracellular ATP, together with the cognate receptors on the cell surface, ligand-gated ion channel P2X receptors and a subset of G-protein-coupled P2Y receptors, represents common autocrine and/or paracrine Ca2+ signalling mechanisms. The P2X receptor ion channels mediate extracellular Ca2+ influx, whereas stimulation of the P2Y receptors triggers intracellular Ca2+ release from the endoplasmic reticulum (ER), and activation of both type of receptors thus can elevate the cytosolic Ca2+ concentration ([Ca2+]c), albeit with different kinetics and capacity. Reduction in the ER Ca2+ level following the P2Y receptor activation can further induce store-operated Ca2+ entry as a distinct Ca2+ influx pathway that contributes in ATP-induced increase in the [Ca2+]c. Mesenchymal stem cells (MSC) are a group of multipotent stem cells that grow from adult tissues and hold promising applications in tissue engineering and cell-based therapies treating a great and diverse number of diseases. There is increasing evidence to show constitutive or evoked ATP release from stem cells themselves or mature cells in the close vicinity. In this review, we discuss the mechanisms for ATP release and clearance, the receptors and ion channels participating in ATP-induced Ca2+ signalling and the roles of such signalling mechanisms in mediating ATP-induced regulation of MSC migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell migration from one location to another is fundamental to diverse physiological processes ranging from tissue morphogenesis and homeostasis to wound healing and immune surveillance and also to pathological processes such as cancer cell invasion [1,2,3,4,5,6]. Cell migration is a complex and highly coordinated process. Adhesive cells often migrate in the so-called mesenchymal mode, in which the migrating cell undergo rear-to-front polarization, protrusion and adhesion formation, and rear retraction. All these major steps in cell migration are orchestrated by numerous scaffold, adaptor and adhesion proteins (e.g., actin, myosin, integrin, paxillin and tensin) in concerted actions that are regulated by various signalling molecules, including protein kinase C (PKC), mitogen-activated protein kinases [MAPK; c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38], Rho GTPase, Rho kinase, and focal adhesion kinase [1, 7,8,9]. As the ubiquitous second messenger, cytosolic Ca2+ plays an important role in regulating many cell functions, including cell migration, in response to diverse physical, chemical and biological clues from the surrounding environments [10,11,12,13,14,15,16,17,18,19,20].
Stem cells are a group of specialized cells resident in several tissues or organs in the body. They are endowed with two unique abilities, namely, self-renewal and differentiation. Embryonic stem cells from the inner cell mass of the pre-implantation blastocyst are pluripotent and give rise to almost every cell type, whereas adult stem cells are multipotent and differentiate to the cell types for the tissue or organ in which they reside and, for this reason, these cells are also referred to tissue-specific stem cells. To date, several types of adult stem cells have been identified. For example, hematopoietic stem or progenitor cells (HSC/HPC) in the bone marrow can give rise to all blood cell types, and the bone marrow transplantation is a hematopoietic stem cell-based therapy for diseases like leukaemia, multiple myeloma and lymphoma [21]. Neural stem or progenitor cells (NSC/NPC) are found in the two major neurogenic niches in the brain, the subventricular zone of the lateral ventricle and the subgranular zone within the dentate gyrus of hippocampus. They have the potential of differentiating to neuron, astrocyte and oligodendrocyte, three major cell types in the nervous system and, therefore, are critical in neurogenesis [22]. Cardiac stem or progenitor cells (CSC/CPC) in the heart can generate myocyte, smooth muscle and endothelial cell [23, 24]. Mesenchymal stem cells or multipotent stromal cells (MSC), present in the connective tissue that surrounds other tissues and organs, exhibit differentiation into multiple cell types, including osteoblast, adipocyte, chondrocyte, and potentially muscle cell, myocyte, neuron and glial cell [25,26,27,28]. MSC can be easily isolated from several adult tissues, readily expanded in vitro, and exhibit robust immunomodulatory properties. All these highly desirable attributes make MSC to be a stem cell source in the development of regenerative medicines. Indeed, a huge number of preclinical studies have demonstrated promising therapeutic applications of MSC in tissue engineering and cell-based therapy to repair and replace damaged or lost cells and tissues due to a variety of injury or diseases including autoimmune disorders [25, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The migrating or homing ability of stem cells to the destined tissues or lesions is not only crucial for normal tissue morphogenesis, homeostasis and repair, but also for development of stem cell-based regenerative medicines [46,47,48,49,50,51,52,53,54]. There is accumulating evidence to show the importance of Ca2+ signalling mechanisms in the regulation of both embryonic and adult stem cell migration [43, 48, 50, 54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70].
ATP is known as the major cellular energy source present at high concentrations inside every living cell, and thus inevitably appears extracellularly at, and in the close vicinity to, the site of tissue damage or inflammation. The ancient and universal availability of ATP prompts the interesting idea that this molecule likely represents the first extracellular signal and purinergic signalling is the primordial form of cell-to-cell communications in multi-cell organisms [71]. Regardless, it has become clear nowadays that in addition to cytolytic leakage from damaged or dying cells, ATP is released via non-cytolytic mechanisms from many cell types and, once outside the cell, it acts as an autocrine and/or paracrine signalling molecule by elevating the cytosolic Ca2+ concentration ([Ca2+]c) via activating the ionotropic P2X receptors and metabotropic P2Y receptors on the cell surface. There is increasing evidence to suggest that ATP-induced purinergic signalling gives rise to significant effects on stem and progenitor cell proliferation, migration and differentiation under in vitro and in vivo conditions [47, 55, 57, 69, 72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. In this short review, we aim to give an overview of ATP-induced Ca2+ signalling mechanisms mainly in MSC and briefly on other adult stem and progenitor cells. We start with an introduction of ATP release and clearance, and then discuss the receptors and ion channels participating in ATP-induced Ca2+ signalling and the role of such signalling mechanisms in ATP-induced regulation of cell migration.
ATP release and clearance
An earlier study examining the Ca2+ signalling mechanisms responsible for the spontaneous oscillations in the [Ca2+]c in NSC/NPC derived from embryonic striatum provided the first clue that stem cells can release ATP [56]. The spontaneous Ca2+ oscillations were prevented by treatment with apyrase, an ecto-enzyme that, as discussed further below, catalyzes hydrolysis of extracellular ATP. Such a finding strongly suggests constitutive release of ATP as part of the mechanisms generating spontaneous Ca2+ oscillations. Spontaneous Ca2+ oscillations were later on documented in a subset of human bone marrow-derived MSC (BM-MSC) [79]. Such spontaneous Ca2+ oscillations in human BM-MSC were largely halted in the extracellular solution containing glucose, but remained in the glucose-free solution, upon treatment with hexokinase, an enzyme that uses ATP to phosphorylate glucose [79]. Measurement of the ATP content in the cell culture medium using the luciferin/luciferase assay showed that a significant amount of ATP was present in the medium culturing human BM-MSC but not in the cell-free medium [79]. These findings support that human BM-MSC can constitutively release ATP. A subsequent study, also by measuring the concentrations of ATP in the cell culture medium, provided independent evidence to confirm constitutive release of ATP from human BM-MSC under in vitro culturing conditions [76]. There is evidence that human BM-MSC can also constitutively release β-nicotinamide adenine dinucleotide (β-NAD) [60]. ATP release from human BM-MSC was robustly enhanced by mechanical stimuli, such as fluid flow-induced shear stress [77] or shockwave [78]. Similarly, β-NAD release from human BM-MSC was stimulated in response to fluid flow-induced shear stress [60].
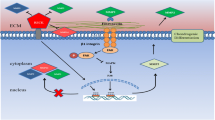
Constitutive or evoked ATP release has been well documented in many mature cell types, but the underlying mechanisms still remain not fully defined. Studies have shown that exocytosis or secretion of ATP from ATP-containing vesicles in the presynaptic neurons as a co-neurotransmitter or neuromodulator [92, 93] and from ATP-containing lysosome in microglial cells [94] occurs in the central and peripheral nervous systems. Additional molecular mechanisms for ATP release have been proposed, including diffusional movement through a diversity of ion channels and transporters, such as connexin (Cx) or pannexin hemi-channels, cystic fibrosis transmembrane conductance regulator, volume-regulated Cl− channel, P2X7 receptor ion channel, and multidrug resistance transporter [92, 93, 95,96,97,98]. Evidence also exists to suggest that the same cells are equipped with multiple ATP release mechanisms and the mechanism used may depend on the situations with which the cells encounter. BM-MSC is one example (Fig. 1). Constitutive ATP release from human BM-MSC cultured in vitro was strongly inhibited by treatment with octanol, palmitoleic acid or 18-α-glycyrrhetinic acid, which are known blockers of the Cx hemi-channels. Such results consistently support a critical role for the Cx hemi-channels in mediating constitutive ATP release [79]. Furthermore, it has been shown that the Cx43 hemi-channel mediates ATP release from pigment epithelium cells [99, 100], whereas the Cx45 hemi-channel serves as the route of ATP release from neural progenitor cells [97]. There is evidence that the Cx43 hemi-channel is functionally expressed in human BM-MSC and plays an important role in mediating constitutive release of β-NAD [60]. However, it remains unclear whether it is involved in constitutive release of ATP. In contrast with constitutive release of ATP, shear stress-induced ATP release from human BM-MSC was insensitive to blockage by 18-α-glycyrrhetinic acid, and instead strongly suppressed by treatment with monensin, an inhibitor of vesicular transport, or treatment with N-ethylmalemide to block the fusion of vesicle to the plasma membrane, therefore favouring the notion that ATP is released in response to shear stress via vesicular exocytosis [77]. There is evidence that vesicular release of ATP is Ca2+-dependent [57, 94]. However, it is unclear whether shear stress-induced vesicular release of ATP from human BM-MSC is Ca2+-dependent and, if it is the case, which Ca2+ signalling mechanism is involved. Several recent studies have demonstrated that the newly-discovered Ca2+-permeable, mechanosensitive Piezo1 channel plays a critical role in mediating stretch or stress-evoked Ca2+ influx and ATP release in urothelial cells [101], red blood cells [102] and endothelial cells [103]. An electrophysiological study has recently reported functional expression of a mechanosensitive stretch-activated Ca2+-permeable channel in human MSC derived from desquamated endometrium in menstrual blood, but the molecular identity of this channel has not been established [104]. Therefore, it is interesting to examine whether the Piezo1 channel is expressed in human MSC and plays a role in mechanical stimuli-induced ATP release as well as mechanical stimuli-induced regulation of MSC proliferation and differentiation [77, 78, 105].
Schematic diagram illustrating the molecular mechanisms for ATP release and hydrolysis, and ATP-induced Ca2+ signalling in MSC. MSC release ATP constitutively through connexin (Cx) hemi-channels and in response to mechanical stimuli via vesicular exocytosis. Extracellular ATP are hydrolyzed to ADP and AMP by ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) and ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) and further to adenosine (Ade) by ecto-5′-nucelotidase (E-NT). Once outside the cell, ATP acts as an autocrine or paracrine signalling molecule by elevating the cytosolic Ca2+ concentrations ([Ca2+]c) through three molecular mechanisms. ATP can induce activation of the P2X7 receptor ion channel allowing extracellular Ca2+ influx. Alternatively, ATP can activate the P2Y1, P2Y2 and/or P2Y11 receptor, leading to sequential activation of Gα,q/11, phospholipase C (PLC), conversion of membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG), activation of the receptor for IP3 (IP3R) and Ca2+ release from the endoplasmic reticulum (ER). Depletion of the ER Ca2+, upon activation of the Gα,q/11-PLC-IP3R signalling pathway or blockage of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) induces extracellular Ca2+ entry via the store-operated Ca2+ (SOC) or Ca2+ release activated Ca2+ (CRAC) channel. Stim1 acts as the ER Ca2+ sensor via the EF-hand motifs located in the ER lumen (denoted by yellow strip) to monitor the ER Ca2+ level. Reduction in the ER Ca2+ level induces conformal changes in Stim1, leading to its translocation to and trapping at the ER-plasma membrane junction, where it interacts with the Orai1 protein to open the Ca2+-permeating channel. Further details and references are described in the text. The structural features of the P2X7 receptor, Orai1 channel and P2Y receptor are illustrated on the right
In addition to being a physiological signal, extracellular ATP is known as a danger signal because a large quantity of ATP efflux can cause tissue damage and inflammation. Thus, almost all cell types have equipped with some capacity of terminating the action of ATP, particularly protecting against ATP-induced damage, by expressing ecto-nucleotidases that catalyse ATP hydrolysis. Members of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family, such as E-NTPDase 1 (ecto-apyrase or CD39) and NTPDase 2 (ecto-ATPase or CD39L1), and the ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP) family represent the major ecto-enzymes that degrade ATP to ADP and AMP, which is further converted to adenosine by ecto-5′-nucelotidase (Fig. 1) [106]. MSC express multiple ecto-nucleotidases, for example, ecto-apyrase in human BM-MSC detected using immunocytochemistry [107] and human MSC derived from gingival tissues in immune-labelling and cell sorting analysis [42], and NPP1 and NPP3 in human BM-MSC shown at the mRNA level using reverse transcription-polymerase chain reaction (RT-PCR) [60]. Consistently, a recent study has shown that the concentrations of ATP released from human BM-MSC into the culture medium in response to shockwave was several-fold higher after suramin was included in the medium to inhibit ecto-nucleotidases and thereby prevent ATP hydrolysis [78].
Role of P2X receptors in ATP-induced Ca2+ signalling
ATP-induced Ca2+ responses have been documented in various MSC preparations from human BM, adipose tissues (AT), umbilical cord (UC), periodontal ligament (PDL) and dental pulp (DP) as well as from rat BM and AT (Table 1). ATP-induced increase in the [Ca2+]c in the extracellular Ca2+-containing solutions was often biphasic, comprising an initial transient component and a sustained component, as shown in human BM-MSC [50, 108] and human DP-MSC [109]. The sustained Ca2+ response component was largely lost or significantly attenuated in the extracellular Ca2+-free solutions, indicating that Ca2+ influx is predominantly responsible for such ATP-induced increase in the [Ca2+]c.
The P2X receptors are ligand-gated Ca2+-permeable cation channels that are activated by extracellular ATP and therefore can mediate ATP-induced Ca2+ influx. Mammalian cells express seven genes encoding seven P2X receptor subunits, P2X1-P2X7, with a membrane topology consisting of intracellular N-/C-termini, and two α-helical transmembrane segments (TM1 and TM2) connected by a large extracellular domain (Fig. 1) [110,111,112,113]. They form homo-trimers or hetero-trimers, in which ATP binding at the inter-subunit interface of the extracellular part induces conformational changes leading to opening of the Ca2+-permeating pathway formed by the TM2 segment from each of the three subunits that allows entry of extracellular Ca2+ into the cell to elevate the [Ca2+]c [114].
Coppi et al. were the first to show by whole-cell patch-clamp current recording that ATP elicited an inwardly-rectifying current with a reversal potential of ~0 mV in a subset of human BM-MSC [76], providing direct evidence to demonstrate the expression of functional P2X receptor. As highlighted in our recent review, there is noticeable discrepancy in the findings reported by previous studies in terms of the P2X receptor expression at the mRNA, protein and functional levels in MSC derived from different tissues and species [26]. Several studies, using Ca2+ imaging, investigated the role of the P2X receptors in mediating ATP-induced Ca2+ signalling and, particularly aimed to identify the P2X receptor type that participates in such ATP-induced Ca2+ signalling, in combination with pharmacological and/or genetic means. Thus, ATP-elicited increase in the [Ca2+]c in human AT-MSC was prevented by treatment with suramin, a P2 receptor generic antagonist, and attenuated by NF279 [115]. NF279 is known as the P2X1 receptor antagonist with a nanomolar potency but, at the high concentration (100 µM) used in the study, it can also inhibit several other P2X receptors. It is therefore difficult to conclude whether the P2X1 receptor is involved in ATP-induced Ca2+ signalling but, nonetheless, these results are conistent with the expression of functional P2X receptors and contribution in ATP-induced Ca2+ signalling. An earlier study showed that chondrogenic differentiation of mouse BM-MSC was suppressed by treatment with 5-BDBD, a P2X4 receptor selective antagonist, leading the author to put forth that the P2X4 receptor is functionally expressed and mediates ATP-induced Ca2+ influx in mouse BM-MSC [75]. In a recent study, we show the P2X4 mRNA expression in human DP-MSC using RT-PCR. However, ATP-induced increase in the [Ca2+]c was insensitive to blockage by 5-BDBD, failing to support a significant role for the P2X4 receptor in ATP-induced increase in the [Ca2+]c [109].
As summarized in Table 1, several independent studies provide consistent evidence to indicate an important role for the P2X7 receptor in ATP-induced Ca2+ signalling in human MSC. Expression of the P2X7 receptor was consistently demonstrated at the mRNA and/or protein levels in human BM-MSC [50, 78, 108], AT-MSC [115], PDL-MSC [116] and DP-MSC [109]. The sustained component of ATP-evoked increase in the [Ca2+]c in human BM-MSC was almost completely abolished by treatment with KN62 [50], a human P2X7 receptor selective antagonist. It is well-known that 2′,3′-(benzoyl-4-benzoyl)-ATP (BzATP) is more potent than ATP at the P2X7 receptors and that prolonged activation of the P2X7 receptor induces large pore formation and membrane blebbing, which represent the signature characteristics of the P2X7 receptor activation [117,118,119]. Both ATP and BzATP evoked large pore formation and membrane blebbing in human BM-MSC as well as sustained increase in the [Ca2+]c and, in addition, BzATP was more potent than ATP in inducing these responses [108]. Furthermore, BzATP-induced responses were inhibited by treatment with A-438079 [108], a P2X7 receptor selective antagonist. In human PDL-MSC, BzATP was also effective in increasing the [Ca2+]c and inducing the large pore formation, both of which were inhibited by treatment with oxidized ATP (oxATP), an irreversible P2X7 receptor inhibitor [116]. In human DP-MSC, ATP (0.3–300 μM) induced concentration-dependent increases in the [Ca2+]c with a half-maximum concentration (EC50) of approximately 20 μM [109]. ATP-induced Ca2+ responses were attenuated by treatment with AZ11645373, a human P2X7 receptor specific antagonist. BzATP also induced concentration-dependent increases in the [Ca2+]c in human DP-MSC. The maximal Ca2+ response amplitude induced by BzATP was greater than that induced by ATP. Furthermore, BzATP/ATP-induced Ca2+ responses were attenuated by treatment with P2X7-specific siRNA [109]. Collectively, these studies have provided strong evidence to support the expression of functional P2X7 receptor and significant contribution in ATP-induced Ca2+ signalling in human MSC. Another recent study shows mRNA and protein expression of P2X7 receptor in rat BM-MSC [120]. Moreover, BzATP-induced regulation of adipogenic and osteogenic differentiation was attenuated by treatment with brilliant blue G, a P2X7 receptor selective antagonist, or after siRNA-mediated knockdown of the P2X7 receptor expression. These findings consistently support the expression of functional P2X7 receptor and further demonstrate its important role in the regulation of MSC differentiation [120]. However, it is worth mentioning the study examining the role of the P2X receptors in ATP-induced Ca2+ signalling in rat AT-MSC [121]. In this study, the mRNA transcript was detected for the P2X3 and P2X4, but not the P2X7 and any other P2X subunits. ATP (10–1000 μM) also induced concentration-dependent increases in the [Ca2+]c, but ATP-induced increase in the [Ca2+]c was not affected by treatment with AZ10606120 [121], a P2X7 receptor selective antagonist, thus contradicting the notion that the P2X7 receptor mediates ATP-induced increase in the [Ca2+]c. The exact reason for such discrepancy in terms of the P2X7 receptor expression in rat BM-MSC and AT-MSC preparations remains unclear.
Role of P2Y receptors in ATP-induced Ca2+ signalling
The P2Y receptors are distinguished from the P2X receptors in their structural and pharmacological properties and also the signalling mechanisms they mediate [122]. First of all, the P2Y receptors belong to the G-protein-coupled receptor superfamily with a membrane topology made of seven α-helical membrane-spanning segments, extracellular N-terminus and intracellular C-terminus (Fig. 1) [123]. Secondly, the P2Y receptors are different from the P2X receptors in their sensitivity to extracellular nucleotides [124,125,126,127,128]. There are eight mammalian P2Y receptor types, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14. While all the P2X receptors are exclusively activated by ATP, the P2Y receptors are activated by various nucleotides with a highly different potency or sensitivity. For example, the human P2Y receptors have the following agonist profile: P2Y1 (ADP > ATP), P2Y2 (UTP ≥ ATP), P2Y4 (UTP), P2Y6 (UDP > UTP), P2Y11 (ATP > UTP), P2Y12 (ADP > ATP), P2Y13 (ADP > ATP) and P2Y14 (UDP~UDP-glucose) [125]. The third major difference lies in the signalling mechanisms. The P2Y receptors are coupled with various Gα subunits and downstream signalling pathways. More specifically, the P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors (or the P2Y1-like receptors; [125]) are linked with the Gα,q/11, and activation of these receptors leads to sequential activation of phospholipase C (PLC) β, generation of inositol triphosphate (IP3), activation of IP3 receptor (IP3R) and IP3R-mediated Ca2+ release from the endoplasmic reticulum (ER). Therefore, activation of the Gα,q/11-PLC-IP3R signalling pathway can increase the [Ca2+]c via triggering Ca2+ release from intracellular stores (Fig. 1). Stimulation of the P2Y11 receptor can additionally activate adenylyl cyclase (AC) and promote generation of cyclic adenosine monophosphate (cAMP) via association with the Gα,s. By contrast, the P2Y12-P2Y14 receptors (or the P2Y12-likeeceptors; [125]) are coupled to the Gα,i and activation of these receptors inhibits the AC activity and cAMP generation.
Expression of the P2Y receptors has been examined in human BM-MSC, AT-MSC and DP-MSC, and also rat and mouse BM-MSC at the mRNA, protein and/or functional levels. As introduced above, the P2Y1, P2Y2 and P2Y11 receptors represent the major ATP-sensitive human P2Y receptors that are coupled to the Gα,q/11-PLC-IP3R signalling pathway triggering intracellular Ca2+ release. Therefore, our discussion below is confined to the studies examining expression of these three receptors and their role in ATP-induced Ca2+ signalling. Two recent reviews provide further details regarding expression of the P2Y receptors and function in MSC [26, 129]. As discussed above, human BM-MSC [50, 108] and human DP-MSC [109] showed biphasic Ca2+ responses to ATP in extracellular Ca2+-containing solutions. The initial transient increase in the [Ca2+]c largely remained in extracellular Ca2+-free solutions, indicating ATP-induced Ca2+ release from intracellular stores. Such observations provide unambiguous evidence to show the expression of ATP-sensitive P2Y receptors that are coupled to the Gα,q/11-PLC-IP3R signalling pathway. An early study by Kawano et al. showed that the spontaneous Ca2+ oscillations were prevented in a large proportion of human BM-MSC by treatment with BzATP, adenosine 3′-phosphate 5′-phosphosulfate or pyridoxal phosphate-6-azo (benzene-2,4-disulfonic acid) (PPADS). These observations led to the proposal that activation of the P2Y1 receptor is an essential step in an autocrine/paracrine feedback mechanism sustaining the spontaneous Ca2+ oscillations, namely, activation of the P2Y1 receptor gives rise to sequential activation of the Gα,q/11-PLC-IP3R signaling pathway, IP3R-mediated Ca2+ release from the ER, and induction of the store-operated Ca2+ entry (discussed below in more detail), and an increase in the [Ca2+]c triggers ATP release through the Cx hemi-channels [79] (Fig. 1). Such a signalling mechanism was further supported by the findings that the spontaneous Ca2+ oscillations were terminated by treatment with U73122, a PLC inhibitor, or 2-aminoethoxydiphenyl borate (2-APB), a cell-permeant IP3R blocker (as discussed below, 2-APB is also known to inhibit numerous plasma membrane Ca2+-permeable channels including store-operated Ca2+ channel), or over-expression of an IP3-binding protein to remove free IP3. In the above-mentioned patch-clamp recording study, ATP induced a Ca2+-depenent outward K+ current as well as a P2X receptor-mediated inward current in an overlapping subset of human BM-MSC [76]. The ATP-induced outward K+ current was prevented by treatment with MRS2179, a P2Y1 receptor selective antagonist, suggesting the expression of functional P2Y1 receptor and also a role for this receptor in mediating ATP-induced Ca2+ signalling. Consistently, a subsequent study, using RT-PCR and western blotting, showed the expression of the P2Y1 receptor at both mRNA and protein levels [50]. Further supporting evidence for the expression of functional P2Y1 receptor in human BM-MSC was provided by the findings that ADP, a P2Y1 receptor selective agonist, and ADPβS, a non-hydrolizable ADP analogue, were potent in elevating the [Ca2+]c in these cells [50, 108]. Expsoure to ATP also elevated the [Ca2+]c in human AT-MSC, and expression of the P2Y1 receptor in these cells was detected at both mRNA and protein levels, using RT-PCR and western blotting, respectively [115]. However, it was not clear whether the P2Y1 receptor was involved in ATP-induced Ca2+ signalling in human AT-MSC [115]. In human DP-MSC, we have recently shown that ADP as well as ATP induced significant increases in the [Ca2+]c and the Ca2+ responses induced by both ADP and ATP were reduced by siRNA-mediated knockdown of the P2Y1 receptor expression, indicating the expression of functional P2Y1 receptor and its contribution in ATP-induced Ca2+ signaling [109].
There is evidence for expression of the P2Y2 receptor in MSC but the expression of this receptor appears dependent on the tissue and species from which MSC were prepared. An early study demonstrated the mRNA and protein expression of P2Y2 receptor in rat BM-MSC [130]. In addition, increases in the [Ca2+]c were induced by ATP and UTP, but not ADP and UDP, in both extracellular Ca2+-containing and Ca2+-free solutions. Such an agonist profile is most consistent with the expression of functional P2Y2 receptor in rat BM-MSC [130], which is supported by a recent study that shows that UTP-induced regulation of differentiation was prevented by treatment with P2Y2-specific siRNA [131]. However, the P2Y2 mRNA expression was at a very low level in human DP-MSC from a 9 year old donor and almost undetectable in cells from two adult donors at the ages of 21 and 32, suggesting that the P2Y2 receptor is unlikely to play a major role in ATP-induced Ca2+ signalling, at least in human DP-MSC [109].
Strong evidence supports the expression of functional P2Y11 receptor in human MSC. In human BM-MSC, β-NAD induced a biphasic increase in the [Ca2+]c in the extracellular Ca2+-containing solutions, and both the transient and sustained components were prohibited by treatment with NF157, a P2Y11 receptor selective antagonist, or siRNA-mediated genetic depletion of the P2Y11 receptor expression [132]. It should be pointed out that β-NAD-induced increase in the [Ca2+]c is mediated by the P2Y11-Gα,s-AC-cAMP signalling pathway that leads to extracellular Ca2+ influx and intracellular Ca2+ release mediated by the L-type voltage-gated Ca2+ channel in the plasma membrane and the ryanodine receptor in the ER, respectively [132]. Consistently with the expression of P2Y11 receptor in human BM-MSC, BzATP, an agonist preferentially activating the P2Y11 receptor among the P2Y receptors, induced transient increase in the [Ca2+]c in the extracellular Ca2+-free solutions, or in the extracellular Ca2+-containing solutions in the presence of the P2X7 receptor inhibitor KN62 [50]. In human DP-MSC, there was abundant mRNA expression of the P2Y11 receptor. Knockdown with siRNA of the P2Y11 receptor expression reduced ATP-induced increase in the [Ca2+]c, supporting a significant role for the P2Y11 receptor in mediating ATP-induced Ca2+ signalling [109].
In summary, despite with some discrepancy, studies have accumulated evidence to support functional expression of the P2Y1, P2Y2 and P2Y11 receptors and their contribution in ATP-induced Ca2+ signaling (Table 1).
Store-operated Ca2+ entry in ATP-induced Ca2+ signalling
Depletion or reduction of the ER Ca2+ level, which can be triggered by ATP or numerous other extracellular signals via activation of their cognate G-protein-coupled receptors that are coupled to the Gα,q/11-PLC-IP3R signalling pathway, can further evoke extracellular Ca2+ influx, which is commonly called the store-operated Ca2+ entry through the store-operated Ca2+ (SOC) channels or Ca2+ release-activated Ca2+ (CRAC) channels [133,134,135]. An increasing number of studies have shown the store-operated Ca2+ entry in mature cells and also in stem and progenitor cells as part of ATP-induced Ca2+ signalling mechanism following activation of the P2Y receptors coupled to the Gα,q/11-PLCβ-IP3R signalling pathway [64, 68, 80, 81, 84, 97, 100, 136]. Two distinct proteins, Stim1 and Orai1, have been identified to be critical in mediating the store-operated Ca2+ entry through the CRAC channel [133, 134]. Stim1 is a single membrane-spanning protein with the extended N- and C-termini residing in the ER lumen and the cytosol, respectively, and serves as the Ca2+ sensor via the N-terminal Ca2+-binding EF-hand motifs to monitor the Ca2+ level in the ER (Fig. 1). Orai1 protein comprises intracellular N-/C-termini and four α-helical transmembrane segments, and forms a hexameric complex with the first α-helical transmembrane segment from each of the six subunits constituting the Ca2+-permeating pathway (Fig. 1) [137]. According to the diffusion-trap model for the store-operated Ca2+ entry, Stim1 undergo conformational changes, upon depletion of the ER Ca2+, that facilitate its translocation to and trapping at the ER-plasma membrane junction, where Stim1 binds to and thereby gates the Orai1 channel to open [135]. Additional homologue proteins, Orai2, Orai3 and Stim2, have been discovered but their role in mediating the store-operated Ca2+ entry remains less well-defined [133, 134].
It has been proposed that the store-operated Ca2+ entry contributes to ATP-induced Ca2+ signalling in MSC (Table 1). As mentioned above, Kawano et al. showed the store-operated Ca2+ entry as an essential part of the autocrine/paracrine feedback mechanism generating spontaneous Ca2+ oscillations in human BM-MSC, triggered by ATP-induced activation of the P2Y1 receptor and Gα,q/11-PLCβ-IP3R signalling pathway and ensuing IP3R-mediated Ca2+ release [79, 138]. In an even earlier study, this group showed store-operated Ca2+ entry, using the Ca2+ add-back protocols that are widely used to record Ca2+ influx in the extracellular Ca2+-containing solutions in cells that are prior treated in the extracellular Ca2+-free solutions with thapsigargin (TG) or cyclopiazonic acid (CPA), inhibitors that specifically block the sarco/endoplasmic Ca2+-ATPase (SERCA) and thereby deplete Ca2+ in the ER (Fig. 1) [138]. In addition, they also performed patch-clamp recording to demonstrate functional expression of a highly Ca2+-selective SOC channel that was activated following treatment with CPA, or acetylcholine (ACh) [138] via activation of the muscarinic ACh receptor 1 (mAChR1) that is coupled to the Gα,q/11-PLC-IP3R signalling pathway and Ca2+ release from the ER [62]. However, the molecular identity of the channel mediating the store-operated Ca2+ entry in human BM-MSC still remains elusive. In human DP-MSC, ATP induced intracellular Ca2+ release in the extracellular Ca2+-free solutions, leading to massive Ca2+ influx upon adding Ca2+ back to the extracellular solutions. We further validated functional expression of the SOC channels, using the Ca2+ add-back protocols and TG. Treatment with titrated concentration of 2-APB, or Synta66, a SOC channel specific blocker, reduced TG-induced activation of the SOC channel. Such treatment also attenuated ATP-induced increase in the [Ca2+]c in the extracellular Ca2+-containing solutions, providing strong evidence to show the store-operated Ca2+ entry to be important part of ATP-induced Ca2+ signalling mechanism. Furthermore, we have documented the mRNA transcripts for Orai1, Stim1 and Stim2 in human DP-MSC by RT-PCR. Knockdown using siRNA of the expression of Orai1 or Stim1, but Stim2, reduced TG-induced store-operated Ca2+ entry and also ATP-induced increase in the [Ca2+]c in the extracellular Ca2+-containing solutions [109]. These results provide the first evidence to show that the Orai1/Stim1 CRAC channel plays an important role in mediating store-operated Ca2+ entry as part of ATP-induced Ca2+ signalling mechanism in human DP-MSC (Fig. 1).
ATP-induced Ca2+ signalling mechanisms in the regulation of cell migration
Studies have shown that extracellular ATP induces or stimulates the migration of mature cells, for example, epithelial cells [139, 140] and microglial cells [94]. In particular, extracellular ATP strongly regulates the migrating capacity of cancer cells, which is a critical determinant of cancer invasion or metastasis giving rise to the high casualty [141], and the P2X7 [142,143,144,145] and P2Y2 receptors [86, 146,147,148,149,150,151] have been shown to play a critical role in mediating such ATP-induced regulation of cancer cell migration. There is accumulating evidence that extracellular ATP influences stem cell and progenitor cell migration [47, 48, 55, 57, 58, 87, 88] and genetic and/or pharmacological manipulations or disease-associated alterations of the P2X receptors, P2Y receptors and/or SOC channels and associated Ca2+ signalling mechanism give rise considerable effects on stem and progenitor cell migration [48, 55, 63,64,65, 91, 152].
Evidence is also emerging to support that extracellular ATP regulates human MSC migration under in vitro conditions and also their homing capability in vivo [50, 60, 109]. Ferrari et al. were first to demonstrate, using the trans-well migration assay, that application of exogenous ATP in the culture medium in the upper chamber increased human BM-MSC migration [50]. Addition of ATP in the culture medium in the lower chamber as a chemotactic signal resulted in no effect on the cell migration, but enhanced the chemotaxis in response to chemokine CXCL-12. However, as shown in a separate study, addition of ATP in the lower chamber accelerated human BM-MSC migration [60]. In the same study, prior treatment with ATP also stimulated cell migration [60]. Furthermore, addition of β-NAD in the upper or lower chamber enhanced cell migration [60]. The increase in cell migration induced by β-NAD was abolished by treatment with NF157, a P2Y11 receptor selective antagonist, and also by treatment with 2′,3′-dideoxyadenosine, an AC inhibitor [60]. These results clearly support critical involvement of the P2Y11-Gα,s-AC-cAMP signalling pathway in stimulation of cell migration by β-NAD. In a recent study, we have shown using time-lapse imaging in combination with the scratch-induced wound healing assay that ATP facilitated human DP-MSC migration [109]. Similar ATP-induced stimulatory effect on human DP-MSC migration was also observed using the trans-well assay [109]. ATP-induced increase in human DP-MSC migration was completely abolished by PPADS, but not affected by CGS15943, a generic inhibitor of adenosine receptors, suggesting that ATP-induced stimulation of cell migration is predominantly mediated by activation of the P2 receptors rather than activation of the adenosine receptors by adenosine, the major by-product of ATP hydrolysis (Fig. 1) [109]. Consistently, ATP-induced increase in human DP-MSC migration was attenuated by treatment with AZ11645373 to inhibit the P2X7 receptor, and also by siRNA-mediated knockdown of the expression of the P2X7, P2Y1 or P2Y11 receptor. Such ATP-induced increase in cell migration was also suppressed by treatment with 2-APB at a concentration that preferentially inhibits the store-operated Ca2+ entry and, more specifically, by siRNA-mediated reduction of the expression of Orai1, Stim1 or both [109]. Taken together, these results provide evidence to show that the major ATP-induced Ca2+ signalling mechanisms discussed above, namely, the P2X7, P2Y1 and P2Y11 receptors and the Orai1/Stim1 channel, participate in ATP-induced stimulation of human DP-MSC migration (Fig. 1).
It is worth making a special note of the study by Ferrari et al. that showed that pretreatment with ATP significantly improved the homing ability of human BMS-MSC after they were planted into immunocompromised mice [50]. Such a finding is therapeutically interesting as it provide the first proof of concept that the in vivo migrating or homing capacity of MSC can be purposely fine-tuned by in vitro priming MSC with ATP.
Concluding remarks and perspectives
Recent studies have made, as discussed above, significant advances in understanding the molecular mechanisms underlying ATP-induced Ca2+ signalling in human MSC. Evidence has emerged to show an important role for such Ca2+ signalling mechanisms in extracellular ATP-induced regulation of MSC migration, but more studies are clearly required to provide a more detailed or mechanistic insight. As introduced above, cell migrates in a complex but well-orchestrated process that is often described in three major steps, establishment of a rear-to-front polarity, protrusion and formation of focal adhesions at the front or leading edge, and retraction from the rear edge [1]. There is evidence that Ca2+ signalling regulates polarization [69, 70] and adhesion formation [19]. However, it remains unknown which of these steps in MSC migration is regulated by the above-discussed ATP-induced Ca2+ signalling. As briefly mentioned above, it is well established that PKC, MAPK and other Ca2+-dependent signalling proteins are important regulators of the cytoskeletal proteins coordinating cell migration [1, 7,8,9]. Studies have shown that Ca2+ activates or modulates these signalling molecules in MSC. For example, internal Ca2+ release triggered by activation of the Gα,q-PLC-IP3R signalling pathway activates the PKC-ERK1/2 signalling pathway in ACh-induced rat BM-MSC migration [62]. There is also evidence that P2X7 receptor activation in MSC leads to activation of the ERK1/2 and JNK signalling pathways in ATP-induced down-regulation of adipogenic differentiation [120] or the p38 signalling pathway in the up-regulation of osteogenic differentiation induced by shockwave or extracellular ATP [78]. Further studies are required to examine whether such Ca2+-dependent signalling pathways are involved in ATP-induced regulation of MSC migration. Finally, the cell migration or the homing capacity of in vitro expanded MSC cultures to the lesion sites is limited but critical for development of regenerative medicines, particularly cell-based therapy. Evidently, more in vivo studies are required to examine whether the improved understanding of the Ca2+ signalling mechanisms underlying ATP-induced regulation of cell migration can be harnessed to improve the low or poor homing capacity of MSC and thereby the efficacy of promising applications of MSC-based tissue engineering and therapies.
References
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302:1704–1709
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10:445–457
Friedl P, Wolf K (2010) Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188:11–19
Petrie RJ, Doyle AD, Yamada KM (2009) Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol 10:538–549
Bear JE, Haugh JM (2014) Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr Opin Cell Biol 30:74–82
Mayor R, Etienne-Manneville S (2016) The front and rear of collective cell migration. Nat Rev Mol Cell Biol 17:97–109
Amano M, Nakayama M, Kaibuchi K (2010) Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67:545–554
Fogh BS, Multhaupt HA, Couchman JR (2014) Protein kinase C, focal adhesions and the regulation of cell migration. J Histochem Cytochem 62:172–184
Huang C, Jacobson K, Schaller MD (2004) MAP kinases and cell migration. J Cell Sci 117:4619–4628
Melchionda M, Pittman JK, Mayor R, Patel S (2016) Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J Cell Sci 212:803–813
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058
Zheng JQ, Poo MM (2007) Calcium signaling in neuronal motility. Ann Rev Cell Dev Biol 23:375–404
Chun J, Prince A (2009) Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J Leuko Biol 86:1135–1144
Howe AK (2011) Cross-talk between calcium and protein kinase A in the regulation of cell migration. Curr Opin Cell Biol 23:554–561
Lovisolo D, Ariano P, Distasi C (2012) Calcium signaling in neuronal motility: pharmacological tools for investigating specific pathways. Curr Med Chem 19:5793–5801
Markova O, Lenne PF (2012) Calcium signaling in developing embryos: focus on the regulation of cell shape changes and collective movements. Semin Cell Dev Biol 23:298–307
Wei C, Wang X, Zheng M, Cheng H (2012) Calcium gradients underlying cell migration. Curr Opin Cell Biol 24:254–261
Zampese E, Pizzo P (2012) Intracellular organelles in the saga of Ca2+ homeostasis: different molecules for different purposes? Cell Mol Life Sci 69:1077–1104
Tsai FC, Meyer T (2012) Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol 22:837–842
Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H (2007) Calcium flickers steer cell migration. Nature 457:901–905
Hoggatt J, Kfoury Y, Scadden DT (2016) Hematopoietic stem cell niche in health and disease. Ann Rev Pathol 11:555–581
Bond AM, Ming GL, Song H (2015) Adult mammalian neural stem Cells and neurogenesis: five decades later. Cell Stem Cell 17:385–395
Barile L, Chimenti I, Gaetani R, Forte E, Miraldi F, Frati G, Messina E, Giacomello A (2007) Cardiac stem cells: isolation, expansion and experimental use for myocardial regeneration. Nat Clin Pract Cardiovasc Med 4(Suppl 1):S9–S14
Leite CF, Almeida TR, Lopes CS, Dias da Silva VJ (2015) Multipotent stem cells of the heart-do they have therapeutic promise? Front Physiol 6:123
Karantalis V, Hare JM (2015) Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 116:1413–1430
Jiang LH, Hao Y, Mousawi F, Peng H, Yang X (2017) Expression of P2 purinergic receptors in mesenchymal stem cells and their roles in extracellular nucleotide regulation of cell functions. J Cell Physiol 232:287–297
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736
Bianco P (2014) “Mesenchymal” stem cells. Annu Rev Cell Dev Biol 30:677–704
Aurrekoetxea M, Garcia-Gallastegui P, Irastorza I, Luzuriaga J, Uribe-Etxebarria V, Unda F, Ibarretxe G (2015) Dental pulp stem cells as a multifaceted tool for bioengineering and the regeneration of craniomaxillofacial tissues. Front Physiol 6
Christ B, Brückner S, Winkler S (2015) The therapeutic promise of mesenchymal stem cells for liver restoration. Trends Mol Med 21:673–686
Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K (2011) Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med 5:146–150
Kim BS, Chun SY, Lee JK, Lim HJ, J-s Bae, Chung H-Y, Atala A, Soker S, Yoo JJ, Kwon TG (2012) Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med 10:94
Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, Wagner ER, Huang E, Gao Y, Gao JL, Kim SH, Zhou JZ et al (2010) Mesenchymal stem cells: molecular characteristics and clinical applications. World J Stem Cells 2:67–80
Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF (2005) Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther 5:1571–1584
Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S (2008) Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26:2201–2210
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 25:2896–2902
Levi B, Longaker MT (2011) Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 29:576–582
Momin EN, Mohyeldin A, Zaidi HA, Vela G, Quinones-Hinojosa A (2010) Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther 5:326–344
Uccelli A, Mancardi G, Chiesa S (2008) Is there a role for mesenchymal stem cells in autoimmune diseases? Autoimmunity 41:592–595
Uccelli A (2008) Adult stem cells for spinal cord injury: what types and how do they work? Cytotherapy 10:541–542
Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG (2013) Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 65:1181–1193
Ke F, Zhang L, Liu Z, Liu J, Yan S, Xu Z, Bai J, Zhu H, Lou F, Wang H, Shi Y, Jiang Y, Su B, Wang H (2014) Autocrine interleukin-6 drives skin-derived mesenchymal stem cell trafficking via regulating voltage-gated Ca2+ channels. Stem Cells 32:2799–2810
Zhu S, Zhang T, Sun C, Yu A, Qi B, Cheng H (2013) Bone marrow mesenchymal stem cells combined with calcium alginate gel modified by hTGF-beta1 for the construction of tissue-engineered cartilage in three-dimensional conditions. Exp Ther Med 5:95–101
Ribeiro TB, Duarte AS, Longhini AL, Pradella F, Farias AS, Luzo AC, Oliveira AL, Olalla Saad ST (2015) Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci Rep 5:16167
Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, Salati S, Salvestrini V, Gulinelli S, Adinolfi E, Ferrari S, Di Virgilio F, Baccarani M, Lemoli RM (2007) The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood 109:533–542
Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM (2012) The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood 120:2365–2375
Liu X, Hashimoto-Torii K, Torii M, Haydar TF, Rakic P (2008) The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci USA 105:11802–11807
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, Kong D (2008) Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 16:571–579
Ferrari D, Gulinelli S, Salvestrini V, Lucchetti G, Zini R, Manfredini R, Caione L, Piacibello W, Ciciarello M, Rossi L, Idzko M, Ferrari S, Di Virgilio F, Lemoli RM (2011) Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exper Hematol 39:360–374, 374 e361–365
Adamiak M, Abdelbaset-Ismail A, Moore JBt, Zhao J, Abdel-Latif A, Wysoczynski M, Ratajczak MZ (2016) Inducible nitric oxide synthase (iNOS) Is a novel negative regulator of hematopoietic stem/progenitor cell trafficking. Stem Cell Rev 13:92–103
Adamiak M, Suszynska M, Abdel-Latif A, Abdelbaset-Ismail A, Ratajczak J, Ratajczak MZ (2016) The involvment of hematopoietic-specific PLC-beta2 in homing and engraftment of hematopoietic stem/progenitor cells. Stem Cell Rev 12:613–620
Ulrich H, Abbracchio MP, Burnstock G (2012) Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev 8:755–767
Moccia F, Lodola F, Dragoni S, Bonetti E, Bottino C, Guerra G, Laforenza U, Rosti V, Tanzi F (2014) Ca2+ signalling in endothelial progenitor cells: a novel means to improve cell-based therapy and impair tumour vascularisation. Curr Vasc Pharmacol 12:87–105
Feng JF, Gao XF, Pu YY, Burnstock G, Xiang Z, He C (2015) P2X7 receptors and Fyn kinase mediate ATP-induced oligodendrocyte progenitor cell migration. Purinergic Signal 11:361–369
Scemes E, Duval N, Meda P (2003) Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci 23:11444–11452
Striedinger K, Meda P, Scemes E (2007) Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia 55:652–662
Striedinger K, Scemes E (2008) Interleukin-1beta affects calcium signaling and in vitro cell migration of astrocyte progenitors. J Neuroimmunol 196:116–123
Santiago MF, Scemes E (2012) Neuroblast migration and P2Y1 receptor mediated calcium signalling depend on 9-O-acetyl GD3 ganglioside. ASN Neuro 4:357–369
Fruscione F, Scarfi S, Ferraris C, Bruzzone S, Benvenuto F, Guida L, Uccelli A, Salis A, Usai C, Jacchetti E, Ilengo C, Scaglione S, Quarto R, Zocchi E, De Flora A (2011) Regulation of human mesenchymal stem cell functions by an autocrine loop involving NAD+ release and P2Y11-mediated signaling. Stem Cell Dev 20:1183–1198
Park JH, Ryu JM, Yun SP, Kim MO, Han HJ (2012) Fibronectin stimulates migration through lipid raft dependent NHE-1 activation in mouse embryonic stem cells: involvement of RhoA, Ca2+/CaM, and ERK. Biochim Biophys Acta 1820:1618–1627
Tang JM, Yuan J, Li Q, Wang JN, Kong X, Zheng F, Zhang L, Chen L, Guo LY, Huang YH, Yang JY, Chen SY (2012) Acetylcholine induces mesenchymal stem cell migration via Ca2+/PKC/ERK1/2 signal pathway. J Cell Biochem 113:2704–2713
Kuang CY, Yu Y, Wang K, Qian DH, Den MY, Huang L (2012) Knockdown of transient receptor potential canonical-1 reduces the proliferation and migration of endothelial progenitor cells. Stem Cell Dev 21:487–496
Wang LY, Zhang JH, Yu J, Yang J, Deng MY, Kang HL, Huang L (2015) Reduction of store-operated Ca2+ entry correlates with endothelial progenitor cell dysfunction in atherosclerotic mice. Stem Cell Dev 24:1582–1590
Xie W, Wang JQ, Wang QC, Wang Y, Yao S, Tang TS (2015) Adult neural progenitor cells from Huntington’s disease mouse brain exhibit increased proliferation and migration due to enhanced calcium and ROS signals. Cell Prolif 48:517–531
Hayashi H, Edin F, Li H, Liu W, Rask-Andersen H (2016) The effect of pulsed electric fields on the electrotactic migration of human neural progenitor cells through the involvement of intracellular calcium signaling. Brain Res 1652:195–203
Zhang L, Yang C, Li J, Zhu Y, Zhang X (2014) High extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 450:1390–1395
Ferreira-Martins J, Rondon-Clavo C, Tugal D, Korn JA, Rizzi R, Padin-Iruegas ME, Ottolenghi S, De Angelis A, Urbanek K, Ide-Iwata N, D’Amario D, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M (2009) Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res 105:764–774
Matta C, Fodor J, Miosge N, Takacs R, Juhasz T, Rybaltovszki H, Toth A, Csernoch L, Zakany R (2015) Purinergic signalling is required for calcium oscillations in migratory chondrogenic progenitor cells. Pflugers Arch 467:429–442
Huang YW, Chang SJ, Harn ICH, Huang HT, Lin HH, Shen MR, Tang MJ, Chiu WT (2015) Mechanosensitive store-operated calcium entry regulates the formation of cell polarity. J Cell Physiol 230:2086–2097
Burnstock G, Verkhratsky A (2009) Evolutionary origins of the purinergic signalling system. Acta Physiol 195:415–447
Biver G, Wang N, Gartland A, Orriss I, Arnett TR, Boeynaems JM, Robaye B (2013) Role of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Stem Cells 31:2747–2758
Riddle RC, Hippe KR, Donahue HJ (2008) Chemotransport contributes to the effect of oscillatory fluid flow on human bone marrow stromal cell proliferation. J Orthop Res 26:918–924
Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, Mittermayr R, Redl H, Junger WG, Sitte HH, Rünzler D (2014) Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem 289:27090–27104
Kwon HJ (2012) Extracellular ATP signaling via P2X4 receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J Endocrinol 214:337–348
Coppi E, Pugliese AM, Urbani S, Melani A, Cerbai E, Mazzanti B, Bosi A, Saccardi R, Pedata F (2007) ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells 25:1840–1849
Riddle RC, Taylor AF, Rogers JR, Donahue HJ (2007) ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. J Bone Miner Res 22:589–600
Sun D, Junger WG, Yuan C, Zhang W, Bao Y, Qin D, Wang C, Tan L, Qi B, Zhu D, Zhang X, Yu T (2013) Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells 31:1170–1180
Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T (2006) ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 39:313–324
Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU (2003) Adenosine triphosphate induces proliferation of human neural stem cells: role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res 72:352–362
Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR (2004) Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43:647–661
Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonte C, Aloisi F, Visentin S (2005) ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev 48:157–165
Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S (2005) Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50:132–144
Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M (2007) Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol 302:356–366
Grimm I, Ullsperger SN, Zimmermann H (2010) Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol 199:181–189
Salvestrini V, Zini R, Rossi L, Gulinelli S, Manfredini R, Bianchi E, Piacibello W, Caione L, Migliardi G, Ricciardi MR, Tafuri A, Romano M, Salati S, Di Virgilio F, Ferrari S, Baccarani M et al (2012) Purinergic signaling inhibits human acute myeloblastic leukemia cell proliferation, migration, and engraftment in immunodeficient mice. Blood 119:217–226
Oliveira SL, Trujillo CA, Negraes PD, Ulrich H (2015) Effects of ATP and NGF on proliferation and migration of neural precursor cells. Neurochem Res 40:1849–1857
Feng W, Wang L, Zheng G (2015) Expression and function of P2 receptors in hematopoietic stem and progenitor cells. Stem Cell Investig 2:14
Mazrouei S, Sharifpanah F, Bekhite MM, Figulla HR, Sauer H, Wartenberg M (2015) Cardiomyogenesis of embryonic stem cells upon purinergic receptor activation by ADP and ATP. Purinergic Signal 11:491–506
Oliveira A, Illes P, Ulrich H (2016) Purinergic receptors in embryonic and adult neurogenesis. Neuropharmacology 104:272–281
Tang Y, Illes P (2017) Regulation of adult neural progenitor cell functions by purinergic signaling. Glia 65:213–230
Pankratov Y, Lalo U, Verkhratsky A, North RA (2006) Vesicular release of ATP at central synapses. Pflugers Archiv 452:589–597
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (2009) Purinergic signalling in the nervous system: an overview. Trends Neurosci 32:19–29
Dou Y, Wu HJ, Li HQ, Qin S, Wang YE, Li J, Lou HF, Chen Z, Li XM, Luo QM, Duan S (2012) Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res 22:1022–1033
Velasquez S, Malik S, Lutz SE, Scemes E, Eugenin EA (2016) Pannexin1 channels are required for chemokine-mediated migration of CD4+ T lymphocytes: role in inflammation and experimental autoimmune encephalomyelitis. J Immunol 196:4338–4347
Makarenkova HP, Shestopalov VI (2014) The role of pannexin hemichannels in inflammation and regeneration. Front Physiol 5:63
Khodosevich K, Zuccotti A, Kreuzberg MM, Le Magueresse C, Frank M, Willecke K, Monyer H (2012) Connexin45 modulates the proliferation of transit-amplifying precursor cells in the mouse subventricular zone. Proc Natl Acad Sci USA 109:20107–20112
Cantiello HF, Jackson GR Jr, Grosman CF, Prat AG, Borkan SC, Wang Y, Reisin IL, O’Riordan CR, Ausiello DA (1998) Electrodiffusional ATP movement through the cystic fibrosis transmembrane conductance regulator. Am J Physiol 274:C799–C809
Pearson RA, Dale N, Llaudet E, Mobbs P (2005) ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46:731–744
Pearson RA, Luneborg NL, Becker DL, Mobbs P (2005) Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci 25:10803–10814
Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M, Tominaga M (2014) Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem 289:16565–16575
Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J (2015) Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci USA 112:11783–11788
Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S (2016) Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 126:4527–4536
Chubinskiy-Nadezhdin VI, Vasileva VY, Pugovkina NA, Vassilieva IO, Morachevskaya EA, Nikolsky NN, Negulyaev YA (2017) Local calcium signalling is mediated by mechanosensitive ion channels in mesenchymal stem cells. Biochem Biophys Res Commun 482:563–568
Riddle RC, Taylor AF, Genetos DC, Donahue HJ (2006) MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol 290:C776–C784
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309
Noronha-Matos JB, Costa MA, Magalhaes-Cardoso MT, Ferreirinha F, Pelletier J, Freitas R, Neves JM, Sevigny J, Correia-de-Sa P (2012) Role of ecto-NTPDases on UDP-sensitive P2Y6 receptor activation during osteogenic differentiation of primary bone marrow stromal cells from postmenopausal women. J Cell Physiol 227:2694–2709
Noronha-Matos JB, Coimbra J, Sa-e-Sousa A, Rocha R, Marinhas J, Freitas R, Guerra-Gomes S, Ferreirinha F, Costa MA, Correia-de-Sa P (2014) P2X7-induced zeiosis promotes osteogenic differentiation and mineralization of postmenopausal bone marrow-derived mesenchymal stem cells. FASEB J 28:5208–5222
Peng H, Hao Y, Mousawi F, Roger S, Li J, Sim JA, Ponnambalam S, Yang X, Jiang LH (2016) Purinergic and store-operated Ca2+ signalling mechanisms in mesenchymal stem cells and their roles in ATP-induced stimulation of cell migration. Stem Cells 34:2102–2114
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Browne LE, Jiang LH, North RA (2010) New structure enlivens interest in P2X receptors. Trends Pharmacol Sci 31:229–237
Khakh BS, North RA (2012) Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76:51–69
Hattori M, Gouaux E (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485:207–212
Mansoor SE, Lu W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E (2016) X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature 538:66–71
Zippel N, Limbach CA, Ratajski N, Urban C, Luparello C, Pansky A, Kassack MU, Tobiasch E (2012) Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cell Dev 21:884–900
Trubiani O, Horenstein AL, Caciagli F, Caputi S, Malavasi F, Ballerini P (2014) Expression of P2X7 ATP receptor mediating the IL8 and CCL20 release in human periodontal ligament stem cells. J Cell Biochem 115:1138–1146
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738
Virginio C, MacKenzie A, North RA, Surprenant A (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol 519:335–346
Wei L, Caseley E, Li D, Jiang LH (2016) ATP-induced P2X receptor-dependent large pore formation: how much do we know? Front Pharmacol 7:5
Li W, Li G, Zhang Y, Wei S, Song M, Wang W, Yuan X, Wu H, Yang Y (2015) Role of P2X7 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Exp Cell Res 339:367–379
Faroni A, Rothwell SW, Grolla AA, Terenghi G, Magnaghi V, Verkhratsky A (2013) Differentiation of adipose-derived stem cells into Schwann cell phenotype induces expression of P2X receptors that control cell death. Cell Death Dis 4:e743
von Kugelgen I, Hoffmann K (2016) Pharmacology and structure of P2Y receptors. Neuropharmacology 104:50–61
Zhang D, Gao ZG, Zhang K, Kiselev E, Crane S, Wang J, Paoletta S, Yi C, Ma L, Zhang W, Han GW, Liu H, Cherezov V, Katritch V, Jiang H, Stevens RC et al (2015) Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520:317–321
Jacobson KA, Balasubramanian R, Deflorian F, Gao Z-G (2012) G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal 8:419–436
von Kügelgen I, Harden TK (2011) Physiology, and Structure of the P2Y Receptors. Pharmacol Purine Pyrimidine Recept 61:373
Burnstock G (2012) Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays 34:218–225
Jacobson KA, Paoletta S, Katritch V, Wu B, Gao ZG, Zhao Q, Stevens RC, Kiselev E (2015) Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol 88:220–230
Jacobson KA, Muller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49
Noronha-Matos JB, Correia-de-Sa P (2016) Mesenchymal stem cells ageing: targeting the “purinome” to promote osteogenic differentiation and bone repair. J Cell Physiol 231:1852–1861
Ichikawa J, Gemba H (2009) Cell density-dependent changes in intracellular Ca2+ mobilization via the P2Y2 receptor in rat bone marrow stromal cells. J Cell Physiol 219:372–381
Li W, Wei S, Liu C, Song M, Wu H, Yang Y (2016) Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: the role of P2Y2 receptor and ERK1/2 signaling. Int J Mol Med 37:63–73
Fruscione F, Scarfì S, Ferraris C, Bruzzone S, Benvenuto F, Guida L, Uccelli A, Salis A, Usai C, Jacchetti E (2010) Regulation of human mesenchymal stem cell functions by an autocrine loop involving NAD+ release and P2Y11-mediated signaling. Stem Cell Dev 20:1183–1198
Parekh AB (2010) Store-operated CRAC channels: function in health and disease. Nat Rev Drug Discov 9:399–410
Amcheslavsky A, Wood ML, Yeromin AV, Parker I, Freites JA, Tobias DJ, Cahalan MD (2015) Molecular biophysics of Orai store-operated Ca2+ channels. Biophys J 108:237–246
Prakriya M, Lewis RS (2015) Store-operated calcium channels. Physiol Rev 95:1383–1436
Jantaratnotai N, Choi HB, McLarnon JG (2009) ATP stimulates chemokine production via a store-operated calcium entry pathway in C6 glioma cells. BMC Cancer 9:442
Hou X, Pedi L, Diver MM, Long SB (2012) Crystal structure of the calcium release-activated calcium channel Orai. Science 338:1308–1313
Kawano S, Shoji S, Ichinose S, Yamagata K, Tagami M, Hiraoka M (2002) Characterization of Ca2+ signaling pathways in human mesenchymal stem cells. Cell Calcium 32:165–174
Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V (2004) P2Y receptors play a critical role in epithelial cell communication and migration. J Cell Biochem 93:1115–1133
Ehring GR, Szabo IL, Jones MK, Sarfeh IJ, Tarnawski AS (2000) ATP-induced Ca2+-signaling enhances rat gastric microvascular endothelial cell migration. J Physiol Pharmacol 51:799–811
Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127:679–695
Jelassi B, Chantome A, Alcaraz-Perez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S (2011) P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 30:2108–2122
Jelassi B, Anchelin M, Chamouton J, Cayuela ML, Clarysse L, Li J, Gore J, Jiang LH, Roger S (2013) Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 34:1487–1496
Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG (2014) P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One 9:e114371
Giannuzzo A, Pedersen SF, Novak I (2015) The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer 14:203
Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX, Fang WG (2013) P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br J Cancer 109:1666–1675
Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S (2013) Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24:130–137
Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, Kim HJ (2014) P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res 16:R77
Jin H, Seo J, Eun SY, Joo YN, Park SW, Lee JH, Chang KC, Kim HJ (2014) P2Y2 R activation by nucleotides promotes skin wound-healing process. Exp Dermatol 23:480–485
Xie R, Xu J, Wen G, Jin H, Liu X, Yang Y, Ji B, Jiang Y, Song P, Dong H, Tuo B (2014) The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem 289:19137–19149
Chadet S, Jelassi B, Wannous R, Angoulvant D, Chevalier S, Besson P, Roger S (2014) The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis 35:1238–1247
Fang J, Chen X, Wang S, Xie T, Du X, Liu H, Wang S, Li X, Chen J, Zhang B, Liang H, Yang Y, Zhang W (2015) The expression of P2X7 receptors in EPCs and their potential role in the targeting of EPCs to brain gliomas. Cancer Biol Ther 16:498–510
Acknowledgements
L-HJ was supported in part by a visiting professorship grant from University François-Rabelais of Tours and Henan Provincial Department of Education (16IRTSTHN020). FM is a recipient of PhD scholarship from the Civil Service Commission of Kuwait Government. The authors are grateful to their postgraduate students and collaborators for the research described in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jiang, LH., Mousawi, F., Yang, X. et al. ATP-induced Ca2+-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell. Mol. Life Sci. 74, 3697–3710 (2017). https://doi.org/10.1007/s00018-017-2545-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2545-6