Abstract
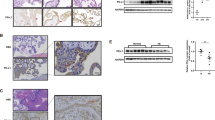
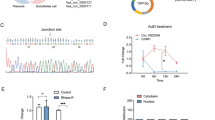
Preeclampsia (PE) is a pregnancy-associated disease, which is the major cause of mortality on maternity and perinatal infants. It is hypothesized that PE is a consequence of the dysfunction of the trophoblast cells. Pleckstrin homology-like domain, family A, member 2 (PHLDA2) was shown to inhibit the proliferation, migration, and invasion of trophoblast cells in our previous studies. However, the mechanism by which PHLDA2 affects trophoblast cell function has not been clarified. In the current study, co-immunoprecipitation (Co-IP) with mass spectroscopy analysis was used to explore the proteins that interacted with PHLDA2. A total of 291 candidate proteins were found to be associated with PHLDA2. The interaction between PHLDA2 and Rho guanine nucleotide dissociation inhibitor (RhoGDI) 1 was identified by Co-IP and immunofluorescence staining. Western blot analysis indicated that overexpression of PHLDA2 resulted in upregulation of the RhoGDI1 protein levels, which were stabilized in the presence of cycloheximide. Similarly, overexpression of RhoGDI1 promoted PHLDA2 expression and its stability. Furthermore, pull-down and Co-IP results indicated that PHLDA2 repressed the activity of Rho guanosine triphosphate hydrolase family proteins by regulating RhoGDI1 expression. In addition, RhoGDI1 expression was upregulated in the placental tissues of patients with PE. The effects of the suppression of PHLDA2 expression on proliferation, migration, and invasion of trophoblast cells were partly abrogated following knockdown of RhoGDI1. Taken together, the data indicated that RhoGDI1 mediated regulation of PHLDA2 on the biological behavior of trophoblast cells and may participate in the pathophysiology of PE.








Similar content being viewed by others
References
Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. 2019;20(17):4246. https://doi.org/10.3390/ijms20174246.
Han C, Han L, Huang P, Chen Y, Wang Y, Xue F. Syncytiotrophoblast-derived extracellular vesicles in pathophysiology of preeclampsia. Front Physiol. 2019;10:1236. https://doi.org/10.3389/fphys.2019.01236.
Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–7. https://doi.org/10.1053/j.semperi.2009.02.010.
Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007. https://doi.org/10.1002/14651858.CD004659.pub3.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–112. https://doi.org/10.1161/CIRCRESAHA.118.313276.
Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972;79(9):794–9.
El-Sayed AAF. Preeclampsia: a review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J Obstet Gynecol. 2017;56(5):593–8. https://doi.org/10.1016/j.tjog.2017.08.004.
Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. https://doi.org/10.1016/j.preghy.2014.02.001.
Phoa KYN, Chedraui P, Pérez-López FR, Wendte JF, Ghiabi S, Vrijkotte T, et al. Perinatal outcome in singleton pregnancies complicated with preeclampsia and eclampsia in Ecuador. J Obstet Gynaecol. 2016;36(5):581–4. https://doi.org/10.3109/01443615.2015.1107532.
Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med. 2019;8(10):1625. https://doi.org/10.3390/jcm8101625.
Frank D, Mendelsohn CL, Ciccone E, Svensson K, Ohlsson R, Tycko B. A novel pleckstrin homology-related gene family defined by Ipl/Tssc3, TDAG51, and Tih1: tissue-specific expression, chromosomal location, and parental imprinting. Mamm Genome. 1999;10(12):1150–9.
Smith AC, Choufani S, Ferreira JC, Weksberg R. Growth regulation, imprinted genes, and chromosome 11p15.5. Pediatr Res. 2007;61(5 Pt 2):43R-R47.
Nagashima T, Shimodaira H, Ide K, Nakakuki T, Tani Y, Takahashi K, et al. Quantitative transcriptional control of ErbB receptor signaling undergoes graded to biphasic response for cell differentiation. J Biol Chem. 2007;282(6):4045–56.
Mullenbrock S, Shah J, Cooper GM. Global expression analysis identified a preferentially nerve growth factor-induced transcriptional program regulated by sustained mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and AP-1 protein activation during PC12 cell differentiation. J Biol Chem. 2011;286(52):45131–45. https://doi.org/10.1074/jbc.M111.274076.
Toriseva M, Ala-aho R, Peltonen S, Peltonen J, Grénman R, Kähäri V-M. Keratinocyte growth factor induces gene expression signature associated with suppression of malignant phenotype of cutaneous squamous carcinoma cells. PLoS ONE. 2012;7(3): e33041. https://doi.org/10.1371/journal.pone.0033041.
Park CG, Lee SY, Kandala G, Lee SY, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;4(6):583–91.
Neef R, Kuske MA, Pröls E, Johnson JP. Identification of the human PHLDA1/TDAG51 gene: down-regulation in metastatic melanoma contributes to apoptosis resistance and growth deregulation. Cancer Res. 2002;62(20):5920–9.
Nagai MA, Fregnani JHTG, Netto MM, Brentani MM, Soares FA. Down-regulation of PHLDA1 gene expression is associated with breast cancer progression. Breast Cancer Res Treat. 2007;106(1):49–56.
Kim HS, Roh CR, Chen B, Tycko B, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of PHLDA2 in primary term human trophoblasts. Placenta. 2007;28(2–3):77–84.
Saxena A, Frank D, Panichkul P, Van den Veyver IB, Tycko B, Thaker H. The product of the imprinted gene IPL marks human villous cytotrophoblast and is lost in complete hydatidiform mole. Placenta. 2003;24(8–9):835–42.
Dória S, Sousa M, Fernandes S, Ramalho C, Brandão O, Matias A, et al. Gene expression pattern of IGF2, PHLDA2, PEG10 and CDKN1C imprinted genes in spontaneous miscarriages or fetal deaths. Epigenetics. 2010;5(5):444–50.
Frank D, Fortino W, Clark L, Musalo R, Wang W, Saxena A, et al. Placental overgrowth in mice lacking the imprinted gene Ipl. Proc Natl Acad Sci USA. 2002;99(11):7490–5.
Salas M, John R, Saxena A, Barton S, Frank D, Fitzpatrick G, et al. Placental growth retardation due to loss of imprinting of Phlda2. Mech Dev. 2004;121(10):1199–210.
Jin F, Qiao C, Luan N, Shang T. The expression of the imprinted gene pleckstrin homology-like domain family A member 2 in placental tissues of preeclampsia and its effects on the proliferation, migration and invasion of trophoblast cells JEG-3. Clin Exp Pharmacol Physiol. 2015;42(11):1142–51. https://doi.org/10.1111/1440-1681.12468.
Jin F, Qiao C, Luan N, Li H. Lentivirus-mediated PHLDA2 overexpression inhibits trophoblast proliferation, migration and invasion, and induces apoptosis. Int J Mol Med. 2016;37(4):949–57. https://doi.org/10.3892/ijmm.2016.2508.
Kuhlmann N, Wroblowski S, Knyphausen P, de Boor S, Brenig J, Zienert AY, et al. Structural and mechanistic insights into the regulation of the fundamental Rho regulator RhoGDIα by lysine acetylation. J Biol Chem. 2016;291(11):5484–99. https://doi.org/10.1074/jbc.M115.707091.
Garcia-Mata R, Boulter E, Burridge K. The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12(8):493–504. https://doi.org/10.1038/nrm3153.
Abramovici H, Mojtabaie P, Parks RJ, Zhong X-P, Koretzky GA, Topham MK, et al. Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell. 2009;20(7):2049–59. https://doi.org/10.1091/mbc.E07-12-1248.
Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17(8):496–510. https://doi.org/10.1038/nrm.2016.67.
Cho HJ, Baek KE, Yoo J. RhoGDI2 as a therapeutic target in cancer. Expert Opin Ther Targets. 2010;14(1):67–75. https://doi.org/10.1517/14728220903449251.
Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93(1):269–309. https://doi.org/10.1152/physrev.00003.2012.
Cho HJ, Hwang YS, Yoon J, Lee M, Lee HG, Daar IO. EphrinB1 promotes cancer cell migration and invasion through the interaction with RhoGDI1. Oncogene. 2018;37(7):861–72. https://doi.org/10.1038/onc.2017.386.
Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12(5):477–83. https://doi.org/10.1038/ncb2049.
Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390(Pt 1):1–9.
Wang J, Zhang L, Qu R, Huang W. Rho A regulates epidermal growth factor-induced human osteosarcoma MG63 cell migration. Int J Mol Sci. 2018;19(5):1437. https://doi.org/10.3390/ijms19051437.
Leabu M, Uniyal S, Xie J, Xu YQ, Vladau C, Morris VL, et al. Integrin alpha2beta1 modulates EGF stimulation of Rho GTPase-dependent morphological changes in adherent human rhabdomyosarcoma RD cells. J Cell Physiol. 2005;202(3):754–66. https://doi.org/10.1002/jcp.20163.
Cho HJ, Kim JT, Lee SJ, Hwang YS, Park SY, Kim BY, et al. Protein phosphatase 1B dephosphorylates Rho guanine nucleotide dissociation inhibitor 1 and suppresses cancer cell migration and invasion. Cancer Lett. 2018;417:141–51. https://doi.org/10.1016/j.canlet.2018.01.002.
Jensen AB, Tunster SJ, John RM. The significance of elevated placental PHLDA2 in human growth restricted pregnancies. Placenta. 2014;35(8):528–32. https://doi.org/10.1016/j.placenta.2014.04.018.
Qian N, Frank D, O’Keefe D, Dao D, Zhao L, Yuan L, et al. The IPL gene on chromosome 11p15.5 is imprinted in humans and mice and is similar to TDAG51, implicated in Fas expression and apoptosis. Hum Mol Genet. 1997;6(12):2021–9.
Piedrahita JA. The role of imprinted genes in fetal growth abnormalities. Birth Defects Res A Clin Mol Teratol. 2011;91(8):682–92. https://doi.org/10.1002/bdra.20795.
Moad AIH, Muhammad TST, Oon CE, Tan ML. Rapamycin induces apoptosis when autophagy is inhibited in T-47D mammary cells and both processes are regulated by Phlda1. Cell Biochem Biophys. 2013;66(3):567–87. https://doi.org/10.1007/s12013-012-9504-5.
Johnson EO, Chang K-H, de Pablo Y, Ghosh S, Mehta R, Badve S, et al. PHLDA1 is a crucial negative regulator and effector of Aurora A kinase in breast cancer. J Cell Sci. 2011;124(Pt 16):2711–22. https://doi.org/10.1242/jcs.084970.
Park E-S, Kim J, Ha T-U, Choi J-S, Soo Hong K, Rho J. TDAG51 deficiency promotes oxidative stress-induced apoptosis through the generation of reactive oxygen species in mouse embryonic fibroblasts. Exp Mol Med. 2013;45:e35. https://doi.org/10.1038/emm.2013.67.
Zhang J, Li T, Ji W, Yu Y, Tan T. Rho GDIalpha modulates rabbit trophoblast stem cell survival and migration. Biol Reprod. 2015;93(6):144. https://doi.org/10.1095/biolreprod.115.132019.
Zhao L, Wang H, Li J, Liu Y, Ding Y. Overexpression of Rho GDP-dissociation inhibitor alpha is associated with tumor progression and poor prognosis of colorectal cancer. J Proteome Res. 2008;7(9):3994–4003. https://doi.org/10.1021/pr800271b.
Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9(17):6432–40.
Ding J, Huang S, Wu S, Zhao Y, Liang L, Yan M, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12(4):390–9. https://doi.org/10.1038/ncb2039.
Xiao Y, Lin VY, Ke S, Lin GE, Lin F-T, Lin W-C. 14-3-3τ promotes breast cancer invasion and metastasis by inhibiting RhoGDIα. Mol Cell Biol. 2014;34(14):2635–49.
Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–80.
Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(9):690–701. https://doi.org/10.1038/nrm2476.
Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–55.
Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273(5272):245–8.
Funding
This study was funded by the Natural Science Foundation of Liaoning Province (2019-MS-07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Ethics approval
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University (Permit No. 2021PS381K).
Informed consent
Patients participating in the study are required to sign a written informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, G., Jin, F. RhoGDI1 interacts with PHLDA2, suppresses the proliferation, migration, and invasion of trophoblast cells, and participates in the pathogenesis of preeclampsia. Human Cell 35, 1440–1452 (2022). https://doi.org/10.1007/s13577-022-00746-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00746-w