Abstract
Objective
The objective of this study was to investigate whether cancer-specific survival in rectal cancer patients is affected by patient-related factors, conditional on radiation treatment.
Methods
Three hundred fifty-nine invasive rectal cancer patients who consented and provided questionnaire data for a population-based case-control study of colorectal cancer in Metropolitan Detroit were included in this study. Their vital status was ascertained through to the population-based cancer registry. Hazard ratios (HR) for cancer-specific and other deaths and 95 % confidence intervals (CIs) were calculated according to selected patients’ characteristics, stratified by radiation status, using joint Cox proportional hazards models.
Results
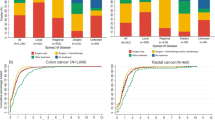
A total of 159 patients were found to be deceased after the median follow-up of 9.2 years, and 70 % of them were considered to be cancer specific. Smoking and a history of diabetes were associated with an increased probability of deaths from other causes (HR 3.20, 95 % CI 1.72–5.97 and HR 2.02, 95 % CI 0.98–4.16, respectively), while regular use of non-steroidal anti-inflammatory drugs (NSAIDs) was inversely correlated with cancer-specific mortality (HR 0.50, 95 % CI 0.30–0.81). Furthermore, the associations of smoking and NSAIDs with the two different types of deaths (cancer vs others) significantly varied with radiation status (P values for the interactions = 0.014 for both). In addition, we observed a marginally significantly reduced risk of cancer-specific deaths in the patients who had the relative ketogenic diet (HR 0.49, 95 % 0.23–1.02).
Conclusion
Further research is warranted to confirm these results in order to develop new interventions to improve outcome from radiation treatment.

Similar content being viewed by others
References
Phang PT, Wang X (2014) Current controversies in neoadjuvant chemoradiation of rectal cancer. Surg Oncol Clin N Am 23(1):79–92. doi:10.1016/j.soc.2013.09.008
Glynne-Jones R, Harrison M, Hughes R (2013) Challenges in the neoadjuvant treatment of rectal cancer: balancing the risk of recurrence and quality of life. Cancer Radiotherapie: journal de la Societe Francaise de Radiotherapie Oncologique 17(7):675–685. doi:10.1016/j.canrad.2013.06.043
Glimelius B (2013) Neo-adjuvant radiotherapy in rectal cancer. World Journal of Gastroenterology: WJG 19(46):8489–8501. doi:10.3748/wjg.v19.i46.8489
Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B (2005) Improved survival in cancer of the colon and rectum in Sweden. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 31(8):845–853. doi:10.1016/j.ejso.2005.05.002
Martijn H, Voogd AC, van de Poll-Franse LV, Repelaer van Driel OJ, Rutten HJ, Coebergh JW (2003) Improved survival of patients with rectal cancer since 1980: a population-based study. European Journal of Cancer (Oxford, England: 1990) 39(14):2073–2079
Spitale A, Franzetti-Pellanda A, Mazzola P, Richetti A, Mazzuchelli L, Bordoni A (2012) Impact of preoperative radiotherapy on survival in locally advanced rectal cancer: an observational population-based study from the south of Switzerland. European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP) 21(2):139–146. doi:10.1097/CEJ.0b013e32834c9c56
Ceelen W, Pattyn P, Boterberg T, Peeters M (2006) Pre-operative combined modality therapy in the management of locally advanced rectal cancer. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 32(3):259–268. doi:10.1016/j.ejso.2005.12.002
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740. doi:10.1056/NEJMoa040694
Kong M, Hong SE, Choi WS, Kim SY, Choi J (2012) Preoperative concurrent chemoradiotherapy for locally advanced rectal cancer: treatment outcomes and analysis of prognostic factors. Cancer Res Treat 44(2):104–112. doi:10.4143/crt.2012.44.2.104
Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC (2012) Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Color Dis 14(10):e701–e707. doi:10.1111/j.1463-1318.2012.03147.x
Toiyama Y, Inoue Y, Saigusa S, Kawamura M, Kawamoto A, Okugawa Y, Hiro J, Tanaka K, Mohri Y, Kusunoki M (2013) C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res 33(11):5065–5074
Meijer TWH, Kaanders JHAM, Span PN, Bussink J (2012) Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res 18(20):5585–5594. doi:10.1158/1078-0432.ccr-12-0858
Toustrup K, Sørensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, Overgaard J (2011) Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 71(17):5923–5931. doi:10.1158/0008-5472.can-11-1182
Semenza Gregg L (2012) Hypoxia-Inducible Factors in Physiology and Medicine. Cell 148 (3):399–408. doi:http://dx.doi.org/10.1016/j.cell.2012.01.021
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10(9):671–684
Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, Conti PS, Chen TC, Longo VD (2012) Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One 7(9):e44603. doi:10.1371/journal.pone.0044603
Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC (2012) The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One 7(5):e36197. doi:10.1371/journal.pone.0036197
Hedblad B, Engström G, Janzon E, Berglund G, Janzon L (2006) COHb% as a marker of cardiovascular risk in never smokers: results from a population-based cohort study. Scandinavian Journal of Public Health 34(6):609–615. doi:10.1080/14034940600590523
Warren GW, Romano MA, Kudrimoti MR, Randall ME, McGarry RC, Singh AK, Rangnekar VM (2012) Nicotinic modulation of therapeutic response in vitro and in vivo. Int J Cancer 131(11):2519–2527. doi:10.1002/ijc.27556
Din FVN, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG (2012) Aspirin Inhibits mTOR Signaling, Activates AMP-Activated Protein Kinase, and Induces Autophagy in Colorectal Cancer Cells. Gastroenterology 142 (7):1504–1515.e1503. doi:http://dx.doi.org/10.1053/j.gastro.2012.02.050
Kato I, Land S, Majumdar AP, Barnholtz-Sloan J, Severson RK (2010) Functional polymorphisms to modulate luminal lipid exposure and risk of colorectal cancer. Cancer Epidemiology 34(3):291–297. doi:10.1016/j.canep.2010.02.010
Kato I, Majumdar AP, Land SJ, Barnholtz-Sloan JS, Severson RK (2010) Dietary fatty acids, luminal modifiers, and risk of colorectal cancer. International Journal of Cancer Journal International du Cancer 127(4):942–951. doi:10.1002/ijc.25103
Willett W, Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124(1):17–27
Xue X, Kim MY, Gaudet MM, Park Y, Heo M, Hollenbeck AR, Strickler HD, Gunter MJ (2013) A comparison of the polytomous logistic regression and joint Cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiology Biomarkers & Prevention 22(2):275–285. doi:10.1158/1055-9965.epi-12-1050
Lunn M, Don M (1995) Applying Cox regression to competing risks. Biometrics 51(2):524–532. doi:10.2307/2532940
Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P (2008) Smoking and colorectal cancer: a meta-analysis. Jama 300(23):2765–2778. doi:10.1001/jama.2008.839
Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB (2008) Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology 134(2):388–395. doi:10.1053/j.gastro.2007.11.007
Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. The Lancet Oncology 10 (11):1033–1034. doi:http://dx.doi.org/10.1016/S1470-2045(09)70326–2
National Center for Chronic Disease P, Health Promotion Office on S, Health (2014) Reports of the Surgeon General. The health consequences of smoking-50 years of progress: a report of the surgeon general. Centers for Disease Control and Prevention (US), Atlanta (GA)
IARC (1997) IARC handbooks of cancer prevention, vol 1. Non-Steroidal Anti-Inflammatory Drugs, IARC, Lyon, France
Algra AM, Rothwell PM (2012) Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The Lancet Oncology 13 (5):518–527. doi:http://dx.doi.org/10.1016/S1470-2045(12)70112–2
Flossmann E, Rothwell PM (2007) Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. The Lancet 369 (9573):1603–1613. doi:http://dx.doi.org/10.1016/S0140-6736(07)60747–8
Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW (2012) Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 379(9826):1602–1612. doi:10.1016/s0140-6736(11)61720-0
Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z (2012) Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379(9826):1591–1601. doi:10.1016/s0140-6736(12)60209-8
Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJM, van Herk-Sukel MPP, Lemmens V, van den Broek CBM, Coebergh JW, Herings RMC, van de Velde CJH, Fodde R, Liefers GJ (2012) Use of Aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer 106 (9):1564–1570. doi:http://www.nature.com/bjc/journal/v106/n9/suppinfo/bjc2012101s1.html
Corvo R, Antognoni P, Sanguineti G (2001) Biological predictors of response to radiotherapy in head and neck cancer: recent advances and emerging perspectives. Tumori 87(6):355–363
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732. doi:10.1038/nrc1187
Semenza GL (2004) Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell 5(5):405–406
Yoo Y-G, Christensen J, Huang LE (2011) HIF-1α confers aggressive malignant traits on human tumor cells independent of its canonical transcriptional function. Cancer Res 71(4):1244–1252. doi:10.1158/0008-5472.can-10-2360
Lu XG, Xing CG, Feng YZ, Chen J, Deng C (2006) Clinical significance of immunohistochemical expression of hypoxia-inducible factor-1alpha as a prognostic marker in rectal adenocarcinoma. Clin Colorectal Cancer 5(5):350–353
Rasheed S, Harris AL, Tekkis PP, Turley H, Silver A, McDonald PJ, Talbot IC, Glynne-Jones R, Northover JM, Guenther T (2009) Hypoxia-inducible factor-1alpha and -2alpha are expressed in most rectal cancers but only hypoxia-inducible factor-1alpha is associated with prognosis. Br J Cancer 100(10):1666–1673. doi:10.1038/sj.bjc.6605026
Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye J, Ni C, Wu P, Wu D, Xu J, Qiu F, Huang J (2013) Prognostic value and clinicopathological differences of HIFs in colorectal cancer: evidence from meta-analysis. PLoS One 8(12):e80337. doi:10.1371/journal.pone.0080337
Silva P, Slevin NJ, Sloan P, Valentine H, Cresswell J, Ryder D, Price P, Homer JJ, West CML (2008) Prognostic significance of tumor hypoxia inducible factor–1α expression for outcome after radiotherapy in oropharyngeal cancer. International Journal of Radiation Oncology Biology Physics 72 (5):1551–1559. doi:http://dx.doi.org/10.1016/j.ijrobp.2008.07.051
Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y (2011) Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncology 47 (2):92–97. doi:http://dx.doi.org/10.1016/j.oraloncology.2010.11.014
Yeung SJ, Pan J, Lee MH (2008) Roles of p53, Myc and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci 65(24):3981–3999. doi:10.1007/s00018-008-8224-x
Garber K (2006) Energy deregulation: licensing tumors to grow. Science 312(5777):1158–1159. doi:10.1126/science.312.5777.1158
Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M, Mueller-Klieser W (2006) Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiotherapy and Oncology 81 (2):130–135. doi:http://dx.doi.org/10.1016/j.radonc.2006.08.012
Woolf EC, Scheck AC (2015) The ketogenic diet for the treatment of malignant glioma. Journal of Lipid Research 56(1):5–10. doi:10.1194/jlr.R046797
Kato I, Yee CL, Dombi GWK, J. F., Severson RK (2007) Race and other factors associated with differential follow-up in a population-based cancer registry. J Registr Manag 34:129–134
Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. 1141–1154. doi:10.1214/aos/1176350951
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509. doi:10.1080/01621459.1999.10474144
Acknowledgments
We thank Ms. Barbara Rusin, Dr. Maria Samerson, and Ms. Ann Bankowski from Wayne State University for technical support and/or collection/selection of blood samples from cases and controls.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study has been approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding sources
This work was supported by Research Grants R01-CA93817 from the National Cancer Institute and R21 DE023181 from the National Institute of Dental and Craniofacial Research and Bridge Funding from Wayne State University.
Rights and permissions
About this article
Cite this article
Kato, I., Dyson, G., Snyder, M. et al. Differential effects of patient-related factors on the outcome of radiation therapy for rectal cancer. J Radiat Oncol 5, 279–286 (2016). https://doi.org/10.1007/s13566-016-0245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-016-0245-8