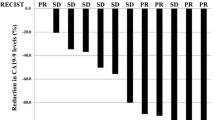
Abstract
Purpose
Potentiation of systemic dosing of gemcitabine using very low-dose fractionated radiotherapy may improve its effect in advanced pancreatic cancer without additional toxicity as suggested by the results of our multi-institutional phase II study. Future regimens combining low-dose fractionated radiotherapy (RT) with systemic chemotherapy should be assessed for improving the chemotherapeutic benefit.
Objective
Until recently, gemcitabine was considered the standard treatment for advanced pancreatic cancer despite low response rates. Efforts to improve response rates by combination therapies have had limited success and have involved using sub-systemic dosing of gemcitabine as a sensitizer for radiation. A novel paradigm, utilizing low-dose fractionated radiotherapy (LDFRT) as a potentiator of full-dose gemcitabine instead, was explored to assess safety and efficacy in treating advanced pancreatic cancer.
Methods
Patients with locally advanced and metastatic pancreatic cancer were treated with 4 cycles of gemcitabine and LDFRT in a multi-institutional phase II study. Gemcitabine (1250 mg/m2 over 2 h) was administered on days 1 and 8 of a 21-day cycle. LDFRT fields included the upper abdomen from the diaphragm to the iliac crest and were administered at 60 cGy per fraction twice daily on days 1, 2, 8, and 9, with the morning fraction being delivered prior to gemcitabine infusion on days 1 and 8. Primary endpoint was objective response rate by Response Evaluation Criteria in Solid Tumors (RECIST).
Results
Thirty-eight patients were enrolled in the trial, of which 23 had metastatic disease at time of enrollment. Objective response rate (CR + PR) was 8 %, and 61 % had at least stable disease. Overall median survival was 13 months and 1-year survival was 37 %. Patients with non-metastatic disease had a 1-year survival of 60 %. The most significant toxicity was hematologic, and only 3 patients were withdrawn from protocol due to toxicity. There was no reported febrile neutropenia.
Conclusion
The use of LDFRT with systemic gemcitabine is safe, tolerable, and potentially effective. In advanced pancreatic cancer, where response rates with single-agent gemcitabine have been low, this chemopotentiation paradigm may improve survival among a poor prognostic patient cohort.


Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A, et al. (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Conroy T, Desseigne F, Ychou M, et al. (2011) Randomized phase III trial comparing FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Fabian C, Giri S, Estes N, et al. (1995) Adjuvant continuous infusion 5-FU, whole-abdominal radiation, and tumor bed boost in the high-risk stage III colon carcinoma: a Southwest Oncology Group pilot study. Int J Radiat Oncol Biol Phys 32(2):457–464
Reddy S, Lee M, Yordan E, et al. (1993) Salvage whole abdomen radiation therapy: its role in ovarian cancer. Int J Radiat Oncol Biol Phys 27:879–884
Eifel P, Gershenson D, Delclos L, et al. (1991) Twice-daily, split-course abdominopelvic radiation therapy after chemotherapy and positive second-look laparotomy for epithelial ovarian carcinoma. Int J Radiat Oncol Biol Phys 21:1013–1018
Corn B, Lanciano R, Boente M, et al. (1994) Recurrent ovarian cancer. Effective radiotherapeutic palliation after chemotherapy failure. Cancer 74:2979–2983
Kunos CA, Sill MW, Buekers TE, et al. (2011) Low-dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: a phase I study of the Gynecologic Oncology Group. Gynecol Oncol 120(2):224–228
Evans DB, Varadhachary GR, Crane CH, et al. (2008) Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 26(21):3492–3502
Magnino A, Gatti M, Massucco P, et al. (2005) Phase II trial of primary radiation therapy and concurrent chemotherapy for patients with locally advanced pancreatic cancer. Oncology 68:493–499
Blackstock AW, Tepper JE, Niedwiecki D, et al. (2003) Cancer and leukemia group B (CALGB) 89805: phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer 34:107–116
Loehrer PJ Sr, Powell ME, Cardenes HR, et al., A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol 26: 2008 (abstract 4506).
Chauffert B, Mornex F, Bonnetain F, et al. (2008) Phase III trial comparing intensive induction chemoradiotherapy (60 cGy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 19(9):1592–1599
Ben-Josef E, Griffith K, Francis IR, et al. (2009) (Abstract 4602) Phase I radiation dose-escalation trial of intensity-modulated radiotherapy (IMRT) with concurrent fixed dose-rate gemcitabine (FDR-G) for unresectable pancreatic cancer. J Clin Oncol 27:15s
Grunewald R, Kantarjian H, Keating MJ, et al. (1990) Pharmacologically directed design of the dose rate and schedule of 2′, 2′-difluorodeoxycytidine (gemcitabine) administration in leukemia. Cancer Res 50(21):6823–6826
Casper ES, Green MR, Kelsen DP, et al. (1994) Phase II trial of gemcitabine (2′, 2′-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Investig New Drugs 12(1):29–34
Carmichael J, Fink U, Russell RC, et al. (1996) Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 73(1):101–105
Burris III HA, Moore MJ, Andersen J, et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Cunningham D, Chau I, Stocken DD, et al. (2009) Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 27(33):5513–5518
Colucci G, Labianca R, DiCostanzo F, et al. (2010) Randomized phase III trial of gemcitabine plus cisplatin compared to single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28(10):1645–1651
Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: a randomized controlled multicentre phase III trial. Lancet Oncol 6(6): 369–376, 2005.
Louvet C, Labianca R, Hammel P, et al. (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23(15):3509–3516
Moore MJ, Goldstein D, Hamm J, et al. (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966
Arnold SM, Regine WF, Ahmed MM, et al. (2004) Low-dose fractionated radiation as a chemopotentiator of neoadjuvant paclitaxel and carboplatin for locally advanced squamous cell carcinoma of the head and neck: results of a new treatment paradigm. Int J Radiat Oncol Biol Phys 58(5):1411–1417
Chendil D, Oakes R, Alcock R, et al. (2000) Low dose fractionated radiation enhances the radiosensitization effect of paclitaxel in colorectal tumor cells with mutant p53. Cancer 89:1893–1900
Jahraus CD, Friedman AH (2010) Chemopotentiation by ultrafractionated radiotherapy in glioblastoma resistant to conventional therapy. Tumori 96:771–775
Joiner MC, Marples B, Labmin P, et al. (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49(2):379–388
Short SC, Joiner MC (1998) Cellular response to low-dose radiation. Clin Oncol 10(2):73–77
Smith LG, Miller RC, Richards M, et al. (1999) Investigation of hypersensitivity to fractionated low-dose radiation exposure. Int J Radiat Oncol Biol Phys 45(1):187–191
Regine WF, Hanna N, Garofalo MC, et al. (2007) Low-dose radiotherapy as a chemopotentiator of gemcitabine in tumors of the pancreas or small bowel: a phase I study exploring a new treatment paradigm. Int J Radiat Biol Phys 68(1):172–177
Braakhuis BJ, van Dongen GA, Vermorken JB, et al. (1991) Preclinical in vivo activity of 2′, 2′-difluorodeoxycytidine (gemcitabine) against human head and neck cancer. Cancer Res 51(1):211–214
Boven E, Schipper H, Erkelens CA, et al. (1993) The influence of the schedule and the dose of gemcitabine on the anti-tumor efficacy in experimental human cancer. Br J Cancer 68(3):52–56
Grunewald R, Abbruzzese JL, Tarassoff P, et al. (1991) Saturation of 2′, 2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol 27(4):258–262
Touroutoglou N, Gravel D, Raber MN, et al. (1998) Clinical results of a pharmacodynamically-based strategy for higher dosing of gemcitabine in patients with solid tumors. Ann Oncol 9(9):1003–1008
Tempero M, Plunkett W, Ruiz van Haperen V, et al. (2003) Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 21:3401–3408
Poplin E, Feng Y, Berlin J, et al. (2009) Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 27(23):3778–3785
Harney J, Short SC, Shah N, Joiner M, Saunders MI (2004) Low dose hyperradiosensitivity in metastatic tumors. Int J Radiat Biol Phys 59(4):1190–1195
Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC (2004) Low-dose hyper-radiosensitivity: a consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat Res 161:247–255
Hall E, Garcia A (2006) Time, dose, and fractionation in radiotherapy. Radiobiology for the radiologist, 6th edition. Lippincott Williams & Wilkins, Philadelphia, pp. 378–397.
Maemura K, Shinchi H, Noma H, et al. (2008) Comparison of hyper-fractionated accelerated and standard fractionated radiotherapy with concomitant low-dose gemcitabine for unresectable pancreatic cancer. Anticancer Res. 28(4C):2369–2372
Reiss KA, Herman JM, Zahurak M, Brade A, Dawson LA, Scardina A, et al. (2015) A phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin Cancer Res. 21:68–76
Funding information
This study was funded by Eli Lilly and Company, 555 12th St. NW, Washington, DC.
Conflict of interest
The authors declare that they have no competing interests.
Statement of ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
This research was performed on a protocol approved by the institutional review board of each of the participating institutions and as per the guidelines for ethical research at each participating institution.
Statement of informed consent
All persons gave their informed consent prior to their inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Participating institutions
University of Maryland, University of Kentucky, Johns Hopkins University, McMaster University.
Prior presentations
Rights and permissions
About this article
Cite this article
Sharma, N.K., Pandya, N.B., Wong, R.K. et al. Evaluation of low-dose fractionated radiation therapy as a chemopotentiator of gemcitabine in advanced pancreatic cancer: results from an international multi-institutional phase II trial. J Radiat Oncol 4, 401–409 (2015). https://doi.org/10.1007/s13566-015-0213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-015-0213-8