Abstract
Background
Tephrosia bracteolata Guill. & Perr. (Leguminosae-Papilionoideae) is a traditional Nigerian medicinal plant used for the treatment of whitlow, toothache, wounds, and diabetes.
Aim
This study investigated the biochemical and histopathological effects of the ethanol extract of T. bracteolata leaves (EETB) and its fractions on alloxan-induced diabetic rats.
Methods
EETB was fractionated successively with n-hexane, chloroform, ethyl acetate, methanol, and water to yield the respective fractions (nHF, CF, EAF, MF, and AF). The antidiabetic activities of EETB and its fractions at 250 and 500 mg/kg body weight (groups 4–15) were investigated in comparison to the normal control (group 1), the diabetic control (group 2), and the standard (150 mg/kg b.w. metformin, group 3) on alloxan-induced diabetic Wistar rats (200–220 g) for 28 days. Alterations in some biochemical parameters and histopathology of major organs were assessed.
Results
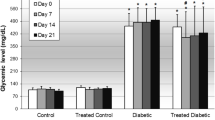
Induction of diabetes triggered significant (p < 0.05) alterations in biochemical indices of the diabetic control relative to the normal control. Following treatments, EAF recorded the most potent effect by restoring altered biochemical parameters examined as indices of diabetic complications. Histopathological examination indicated a rapid regeneration of beta cells, hepatocytes, and nephrotic cells necrotized by alloxan, with EAF producing the best histo-architecture relative to EETB and other fractions.
Conclusions
EAF indicated the most potent antidiabetic effect at the doses investigated, as it reversed complications associated with diabetes in Wistar rats, thus suggesting its potential for future development of potent antidiabetic drugs. Further studies on the characterization of the bioactive principles in EAF are underway.



Similar content being viewed by others
Data availability
Dr. Parker Elijah Joshua and Dr. Precious Adejoh Idakwoji are the curators of the data set, which is available on request.
Abbreviations
- AF:
-
Aqueous fraction
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- BC:
-
Beta cells
- CAT:
-
Catalase
- CF:
-
Chloroform fraction
- CV:
-
Central vacuole
- Dbil:
-
Direct bilirubin
- DM:
-
Diabetes mellitus
- EAF:
-
Ethyl acetate fraction
- EETB:
-
Ethanol extract of Tephrosia bracteolata leaves
- FBS:
-
Fasting blood sugar
- G:
-
Glomerulus
- H:
-
Hepatocytes
- IDF:
-
International Diabetes Foundation
- IST:
-
Islet
- MDA:
-
Malondialdehyde
- MF:
-
Methanol fraction
- nHF:
-
n-Hexane fraction
- RT:
-
Renal tubules
- SOD:
-
Superoxide dismutase
- Tbil:
-
Total bilirubin
- TP:
-
Total protein
- WBC:
-
White blood cell
References
Ekaluo UB, Ikpeme EV, Etta SE, Erem FA, Daniel IO. Protective role of soursop (Annona muricata L.) fruit on testicular toxicity induced by caffeine in albino rats. J Life Sci Res Discov. 2014;1:26–30.
Hutchinson J, Dalziel JM. Flora of West Tropical Africa. London: Crown agents for oversea governments and administrations. 1958;58.
Burkill HM. The useful plants of West Tropical Africa, vol. 1. Kew: Royal Botanical Gardens; 1985. p. 319.
Onaolapo MAO, Nzelibe HC, Aduadi AO, Ayo JO. Toxicity and antipyretic studies of the crude extract of Tephrosia Bracteolata leaves. J Phytomed Ther. 2009;9:91–100.
Egharevba GO, Dosumu OO, Oguntoye SO, Njinga NS, Dahunsi SO, Hamid A, et al. Antidiabetic, antioxidant and antimicrobial activities of extracts of Tephrosia bracteolata leaves. Heliyon. 2019;e02275:5.
International Diabetes Foundation [IDF]. Diabetes atlas. 8th ed. Brussels: International Diabetes Foundation; 2017.
Worldometers.info. Worldometers live counters (based on information from the United Nations document “world population prospects: the 2017 revision”). Retrieved from https://www.worldometers.info.
Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, et al. Prevalence and risk factors for diabetes mellitus in Nigeria: a systematic review and meta-analysis. Diabetes Ther. 2018;9(3):1307–16.
Federiuk IF, Casey HM, Quinn MJ, Wood MD, Ward WK. Induction of type 1 diabetes mellitus in laboratory rats by use of alloxan; route of administration, pitfalls, and insulin treatment. Commun Med. 2009;54:252–7.
Muhammad IU, Jarumi IK, Alhassan AJ, Wudil AM, Dangambo MA. Acute toxicity and hypoglycemic activity of aqueous fruit pulp extract of Adansonia digitata L. (AFPEAD) on alloxan induced diabetic rats. J Adv Med Pharm Sci. 2016;6(3):1–6.
Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N. Reactive aldehydes: second messengers of free radicals in diabetes mellitus. Free Rad Res. 2013;47:39–8.
Derrell C. Guide for care and use of laboratory animals. Institute of Laboratory Animal Resources. Washington DC: National Academy Press; 1996.
Uzor PF, Osadebe PO, Omeje EO, Agbo MO. Bioassay guided isolation and evaluation of the antidiabetic principles of Combretum dolichopetalum root. Br J Pharm Res. 2014;4(18):2155–71.
Trease GE, Evans MC. Pharmacognosy. 13th ed. Tindall: Bailier; 2002.
Eguavoen C, Ekpo DE, Ebeire EN. Effect of seven keys herbal formulation on plasma concentrations of liver transaminases of alloxan-induced diabetic rats. J Pharm Res Int. 2016;11(4):1–11.
Reitman S, Frankel S. Method of alanine and aspartate aminotransferase determination. Am J Clin Pathol. 1957;28:56–8.
Kind PRN, King FJ. Alkaline phosphatase determination. Clin Pathol. 1972;7:322–60.
Bartels H, Bohmer M. In vitro determination of creatinine and urea. Clin Chem. 1972;2:37–193.
Drury RA, Wallington A, Cameroun SR. Carlleton’s histological techniques. 4th ed. London: Oxford University Press; 1967. p. 279–80.
Shayam KP, Kadalmani B. Antidiabetic activity of Bruguiera cylindrical (Linn.) leaf in alloxan-induced diabetic rats. Int J Curr Res Biosci Plant Biol. 2014;1:56–60.
Tefesse TB, Hymete A, Mekonnen Y, Tadesse M. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement Altern Med. 2017;17:243. https://doi.org/10.1186/s12906-017-1757-5.
Morajhar AS, Hardikar B, Sharma B. Hepatoprotective effects of crude extracts of Pongamia pinnata in alloxan-induced diabetic albino Wistar rats. Int J Zoological Res. 2015;11(2):37–8.
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm J. 2016;24(5):547–3.
Obasi E, Ihaenacho K, Nwachukwu N, Agha N, Chikezie PC. Evaluation of body weight, serum glucose level and oxidative stress parameters of diabetic rats administered phenolic aqueous leaf extract of Vitex doniana. Biomed Res Ther. 2019;6(9):3359–67.
Nazir N, Zahoor M, Nisar M, Abdel-Halim H, Ali A. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Complement Altern Med. 2018;18:332. https://doi.org/10.1186/s12906-018-2381-8.
Sindhu RK, Koo JR, Roberts CK, Nosratola D, Vaziri MD. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes response to insulin and antioxidant therapies. Clin Exp Hypertens. 2014;26:43.
Norman OM, Mothana RA, Al-Rehaily AJ, Al Quatani AS, Nasr FA, Khaled JM, et al. Phytochemical analysis and antidiabetic, anti-inflammatory and antioxidant activities of Loranthus acacia Zucc. grown in Saudi Arabia. Saudi Pharm J. 2019;27(5):724–30.
Chu CA, Sherck SM, Igawa K, Sindelar DK, Neal DW, Emshwiller M, et al. Effects of free fatty acids in hepatic glycogenolysis and gluconeogenesis in conscious dogs. Am J Physiol Endocrinol Metab. 2007;282(3):402–11.
Shah D, Nanm DS, Jaishankar GB, Chilakala S, Wang K, Kumaraguru U. Pre-term exposure patterns in neonatal intensive care unit alter immunological outcome in neonates. J Allergy Ther. 2011;2:106. https://doi.org/10.4172/2155-6121.1000106.
Bukonla A, Benson OK, Akinsola AR, Aribigbola C, Adesola A, Seyi A. Effect of type 1 diabetes on serum electrolytes (sodium and potassium) levels and testosterone hormone in human male subjects. Webmedcentral. 2012;3(9):10–1.
Ekpo DE, Joshua PE, Ogidigo JO, Nwodo OFC. High resolution UPLC-PDA-QTOF-ESI-MS/MS analysis of the flavonoid-rich fraction of Lasianthera africana leaves, and in vivo evaluation of its renal and cardiac function effects. Heliyon. 2020;6:e04154. https://doi.org/10.1016/j.heliyon.2020.e04154.
Thakran S, Siddiqui MR, Baquer NZ. Trigonella foenum-graecum seed powder protects against histopathological abnormalities in tissues of diabetic rats. Mol Cell Biochem. 2004;266(1–2):151–9.
Acknowledgments
The authors are thankful to Mr. Onugwu Ernest Okonkwo of the Department of Biochemistry, Salem University, Lokoja, Kogi State, Nigeria, for assisting with animal management and facility procurement. We are also grateful to Mr. Akanni T. Gbenga of the Department of Botany, Federal University, Lokoja, Kogi State, Nigeria, and Mr. Okoyomoh Christian of the Department of Histopathology, Federal Medical Centre, Lokoja, Kogi State, Nigeria, for conducting the microscopic histological assessment.
Author information
Authors and Affiliations
Contributions
P.A. Idakwoji, P.E. Joshua, O.U. Njoku, and O.F.C. Nwodo were responsible for the conceptualization and design of the study. P.A. Idakwoji and D.E. Ekpo conducted the experiments. P.A. Idakwoji, D.E. Ekpo, and P.E. Joshua collected the data set, performed statistical analyses, and interpreted the data. D.E. Ekpo wrote the first manuscript draft and revised it critically for intellectual content. P.E. Joshua, O.U. Njoku, and O.F.C. Nwodo supervised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed involving the use of experimental animals in this study were in accordance with the International Guidelines for Handling of Laboratory Animals [12]. The Institutional Ethics and Biosafety Committee of the Faculty of Biological Sciences, University of Nigeria, Nsukka, Nigeria, with Ethics Committee Approval No.: UNN/FBS/EC/1037.
Consent to participate
Not applicable
Consent for publication
Not applicable
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Idakwoji, P.A., Ekpo, D.E., Joshua, P.E. et al. Ethanol extract of Tephrosia bracteolata leaves and its fractions ameliorates alloxan-induced diabetes and its associated complications in Wistar rat model. Int J Diabetes Dev Ctries 41, 456–468 (2021). https://doi.org/10.1007/s13410-020-00900-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-020-00900-w