Abstract
Background
Diabetes mellitus is a metabolic disorder that affects the body’s ability to produce or use insulin. There is a continuous rise of this disease particularly in developing countries due to changes in life style and poverty among the people. In this study, antidiabetic activities of aqueous extract of Nelsonia canescens and its partitioned fractions in alloxan-induced diabetic rats were evaluated. Male albino rats were divided into 9 groups (diabetic and non-diabetic) of 5 rats each. Diabetes was induced by single intraperitoneal administration of alloxan (90 mg/kgbwt). The experimental design consists of a diabetic control group (untreated), a normal control group (1 mL saline), a standard diabetic drug (Glibenclamide; 5 mg/kgbwt), two doses (50 and 300 mg/kgbwt) of aqueous extract, ethyl acetate and methanol fractions of Nelsonia canescens were orally administered for a period 21 days. Blood glucose of the rats was monitored at 3-days intervals while biochemical and in vivo antioxidant assays of serum and liver were determined after 21 days.
Results
The hypoglycemic effect of the extract observed was in a dose dependent manner with a significant reduction (p < 0.05) of blood glucose in ethylaceate fraction > aqueous extract > methanol fraction compared with the diabetic control group. A significant difference (p < 0.05) in lipid profiles and serum enzyme activity of rats in the diabetic control group was observed compared with the extract and fraction treated groups.
Conclusion
The results suggest that the aqueous extract and fractions of N. canescens showed hypoglycemic and hypolipidemic potentials and significantly (p < 0.05) reduced the progression of oxidative stress.
Similar content being viewed by others
Background
Diabetes mellitus (DM) is a non-communicable endocrine disorder characterized by an alteration in carbohydrate, protein, and fat metabolism [1, 2]. Globally, it is estimated that 463 million people are affected by DM and this figure may rise to 578 million by 2030 and 700 million by 2045 [3, 4]. DM is classified into three types, insulin dependent diabetes mellitus (IDDM) or type 1, non-insulin dependent diabetes mellitus (NIDDM) or type 2 and gestational diabetes mellitus (GDM). IDDM result from total loss of function of β-cell of islets of Langerhans which are present in the pancreas. NIDDM result from a temporary loss of β cell function which may be due to genetic predisposition that mostly occur in obese persons and associated with high blood pressure and high cholesterol levels while gestational diabetes is a type of diabetes with characteristic hyperglycemia in pregnant women in the 2nd or 3rd trimester [3,4,5,6]. Globally, DM is considered the most significant influencer for the development of various clinical conditions such as ischemic heart diseases, peripheral neuropathies, ulcerations, and delayed wound healings which consequently alters the life expectancy of affected patients [7]. Diabetes also affects the amount of lipid in the blood, a condition known as diabetic dyslipidemia [8]. Diabetic patients are characterized with lower serum levels of high-density lipoprotein cholesterol (HDL-C), higher serum levels of triglyceride (TG), and similar serum levels for low-density lipoprotein cholesterol (LDL-C) but with higher levels of small dense LDL when compared with nondiabetic patients [9, 10]. To date, the management of diabetes remains a major global challenge with no recorded success in complete treatment of the disease [2]. Many synthetic medicines have been developed to manage diabetes with no reported evidence of absolute recovery from using these drugs [10,11,12,13,14]. The modern oral hypoglycemic drugs produce undesirable and adverse side effects [2]. Therefore, alternative therapy with improved therapeutic potentials and less side effects is urgently needed [15]. There are many traditional herbal medicines recommended for the treatment and management of diabetes [15]. About 25% of the drugs prescribed across the world are derive from plants already used in traditional medicine [15]. Herbal drugs are prescribed widely because of their effectiveness, less side effects and relatively low cost [11,12,13, 16]. Treatment of DM without any adverse side effects is a major challenge confronting physician [2]. More than 800 medicinal plants are currently used for the treatment and prevention of DM with over 450 that have successfully passed through the clinical trial stage from which 109 have their mode of action elucidated [17,18,19,20]. Traditional medicinal plants with their active constituents are used for treating different kinds of ailments such as heart diseases, cancer, and diabetes since ancient times [2, 20].
Nelsonia canescens (Lam). Spreng of the family Acanthaceae commonly known as the blue pussy plant is a wild medicinal plant which grow abundantly in various parts of the world including India and sub-Saharan Africa [21, 22]. This plant can be found in the rain forest, the savannah and often as secondary colonizers along the road, foot paths and mostly around uninhabited settlements [23]. The plant root is used for the treatment of various inflammatory and pain related ailments such as arthritis [22]. Metabolite analysis of the methanol extract of Nelsonia canescens has reported the presence of phenols, flavonoids, tannins, saponins and alkaloids, whereas steroids, glycoside and phlobatannins were not detected [24]. The aim of this study was to evaluate the antidiabetic activity of N. canescens aqueous extract and its partitioned fractions in alloxan-induced diabetic rats.
Methods
Chemicals and equipment
Gallic acid, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Co., St Louis, USA. Sodium acetate anhydrous and Alloxan Monohydrate were obtained from BDH (BDH Laboratory Supplies, England), Formaldehyde Solution from (Laboratory Rasayan, Boisar); Folin–Ciocalteu phenol reagent (FC) from Merck (KGaA, Germany), Glibenclamide (Daonil; Aventis Pharma. Ltd., India), Cholesterol reagent (Teco Diagnostics., USA), Triglyceride GPO reagent (Teco Diagnostics., USA) and HDL Cholesterol reagent (Agape Diagnostics., Switzerland). Other chemicals were of analytical grade. All spectrophotometric measurements were taken using the double beam Shimadzu UV spectrophotometer, UV-1800 (Shimadzu Scientific Instruments Inc, Nakagyo-ku, Kyoto, Japan) while centrifugation was performed using High Speed Refrigerated Centrifuge, LR10-2.4A (Hunan Kecheng Instrument and Equipment Co., Ltd, Changsha city, China).
Processing and extraction of plant materials
The leaves of Nelsonia canescens (Acanthaceae) were harvested from the vicinity of Bosso campus of Federal University of Technology Minna, Niger State, Nigeria. The plant was identified and authenticated at the Plant Biology Department of Federal University of Technology Minna and a specimen of the plant sample was deposited at the herbarium with a voucher number: FUT/PLB/ACA/001. Plant sample was cleaned and washed with distilled water before air drying at room temperature for 2 weeks before crushing the leaves into smaller particles and blending into fine powder using a clean blender. A portion of the powdered sample (500 g) was refluxed at 40 °C with distilled water for 2 h to obtain the aqueous extract (AQ) which was filtered using a muslin cloth and Whatmann No 1 filter paper. The extract was further concentrated in a water bath at 30 °C and a semi-solid brownish like paste was finally obtained as the extract. The dried extract was stored in the refrigerator at 4 °C prior to usage [23, 25].
Partitioning of the crude extract
Fifty grams (50 g) of the aqueous extract was reconstituted in distilled water and solvent partitioned using ethylacetate (EA) and methanol (ME) in a separating funnel at 25 °C. The fractions were concentrated in a water bath at 30 °C to obtain a solid paste.
Quantitative phytochemical analysis of the extract
Quantitative phytochemical analysis of the crude aqueous extract (AQ), EA and ME fractions were performed using the method described by Chang et al. [26] and Singleton et al. [27].
Total flavonoid content
A colorimetric method using aluminum chloride (AlCl3) was used to quantify the flavonoid content in the crude extract, EA and ME fractions of Nelsonia canescens plants. A 1 mL aliquot of crude extract (1 mg/mL), fractions (EA and ME) or quercetin (12.5–100 mg/mL; standard) were mixed with 0.1 mL of 10% (w/v) AlCl3 in methanol, 0.1 mL of 1 M sodium acetate and 2.8 mL of distilled water. The reaction mixture was incubated at 25 °C for 30 min and absorbance measured at 415 nm against a blank. The results were expressed as mg/g quercetin equivalents.
Total phenolic content
Total phenolic content of the crude extract and fractions (EA and ME) was determined using the Folin–Ciocalteu method. For the crude extract (1 mg/mL) and each fraction, a 0.5 mL aliquot was mixed with 2.5 mL of 10% Folin–Ciocalteu reagent (v/v). This mixture was oxidized with 2 mL of 7.5% Na2CO3 (w/v) and incubated at 45 °C for 40 min. The absorbance was read at 765 nm and the amount of phenols in the crude extract and fractions was calculated using the Gallic acid standard curve expressed as mg/g of gallic acid equivalents.
Alkaloids content
For the measurement of alkaloid content in the crude extract and separate fractions (EA and ME), 0.5 g of each sample was dissolved (separately) in 5 mL of 50% ethanol in sulfuric acid and filtered using Whatmann No 1 filter paper. An aliquot (1 mL) of the filtrate was mixed with 5 mL of 60% H2SO4 (v/v) and incubated for 5 min. After incubation 5 mL of 0.5% of formaldehyde solution (v/v) was added to the sample mixture and incubated for another 3 h and the absorbance measure at 565 nm. The molar extinction coefficient of Vincristine (ɛ = 15,136 mol/cm) was used to estimate the alkaloids content of the crude extract and fractions (EA and ME).
Saponin content
The total saponin content in crude extract and fraction of Nelsonia canescens leaves was determined using a colorimetric assay. 0.5 g of the crude extract and fractions was reconstituted separately in 20 mL of 1 M HCl, at 80 °C for 4 h and then filtered using Whatmann No 1 filter paper. Fifty milliliters of petroleum ether was added to the filtrate in a separating funnel to obtain the ether layer which was evaporated in a water bath. The sample was dissolved in 5 mL of 50% acetone in ethanol, 6 mL ferrous sulfate (FeSO4) reagent and 2 mL of concentrated. H2SO4 were added to it and incubated for 10 min. The absorbance of each sample was measured at 490 nm and the amount of saponins extrapolated from a saponin standard curve.
Tannin acid content
The amount of tannins present in the extract and fraction was calculated using the tannic acid standard curve. 0.2 g each of the crude extract and fractions were weighed separately into a beaker and containing 20 mL of 50% methanol (v/v), covered with a foil paper and placed in a water bath at 80 °C for 1 h. 2.5 and 20 mL Folin–Ciocalteu reagent and distilled water, respectively, were added to the mixture and oxidized with 10 mL of 17% Na2CO3 (w/v). The mixture was incubated at room temperature for 20 min for color development and the absorbance was read at760 nm.
In vitro antioxidant activity of the crude extract and fractions
ABTS radical scavenging assay
This test was based on the samples’ capacity to scavenge the radical cation produced when ABTS solution was reacted with a potent oxidizing agent like potassium permanganate (KMnO4) or potassium persulfate (K2S2O8) [28]. Equal volume of 2.45 mM K2S2O8 and 7.4 mM ABTS stock solution were mixed together to generate the free radicals' persulfate in the dark for 12 to 16 h at room temperature. To get an absorbance of 0.700 ± 0.02 at 734 nm, the ABTS+ solution was diluted with ethanol (0.90 mL ABTS and 25 mL of ethanol). Aliquot of different concentration of the samples (0.1 mL) and 0.9 mL of the ABTS•+ were mixed and allowed to react for 30 min in the dark before reading the absorbance at 734 nm. Percentage inhibition of the radicals was calculated using the formula below:
where A0 and A1 are the absorbance of control (without sample) and test samples, respectively.
DPPH radical scavenging assay
The DPPH radical scavenging potentials of the extract and fractions were evaluated as described by Gyamfi et al. [29]. A total of 1 mL of the crude extract and fractions in a concentration dependent dose (12.5–100 µg/mL) and 1 mL of 0.4 mM methanolic DPPH were mixed and incubated for 30 min in the dark. The absorbance was measured at 516 nm and the percentage inhibition of DPPH radical was calculated relative to the blank (0.4 mM methanolic DPPH) using the equation as follows:
where A0 is the absorbance of control (without sample) and A1 is the absorbance of the extract and DPPH solution.
Experimental animals and ethics
Forty-five (45) male wistar rats (100–150 g) were obtained from the University of Jos animal farm. The rats were acclimatized for 2 weeks at 28 ± 2 °C, 45–55% of relative humidity on a 12 h light/12 h dark cycle, with access to water and pelletized standard guinea feed ad libitum. The rats were kept for 2 weeks to acclimatize to the environmental conditions. The present study was approved by the Ethical Committee on the use of animals for research at the Federal University Technology, Minna, Nigeria with NO: 000018EAU. Handling of the rats was in accordance with the standard principles of laboratory animal care of the United States National Institutes of Health (NIH, 1978) as reported by Belayneh et al. [10].
Induction of diabetes mellitus
A single intraperitoneal (i.p.) injection of 90 mg/kgbwt of alloxan monohydrate (Sigma, St. Louis, USA) in phosphate buffer saline (PBS) with a pH of 7.4 was used to induce diabetes mellitus in the rats. Animals were selected for the study if their fasting plasma glucose concentration (FPGC) was greater than 111 mg/dL for five consecutive days as determined by the Fine test Auto-coding Premium Blood Glucose Monitoring System for self-testing. Nine groups of five male wistar rats each were created from a total of 45 male wistar rats. Before the start of the treatment, the animals were deprived of food and water for an extra 16 h [30].
Experimental design
The experimental design consists of 9 different groups which was based on the treatment received for 21 days as shown as follows:
-
Group I: normal control (non-diabetic) received1 mL PBS
-
Group II: positive control and treated with 5 mg/kgbwt of Glibenclamide (reference drug)
-
Group III: negative control (Diabetic control)
-
Group IV: treated with 50 mg/kgbwt of AQ
-
Group V: treated with 50 mg/kgbwt of EA
-
Group VI: treated with 50 mg/kgbwt of ME
-
Group VII: treated with 300 mg/kgbwt of AQ
-
Group VIII: treated with 300 mg/kgbwt of EA
-
Group IX: treated with 300 mg/kgbwt of ME
The rats were weighed every 7 days to monitor their body weight.
Collection and preparation of blood and tissues
After 21 days, rats who had been fasting overnight were sacrificed by heart puncture anesthetized with 150 mg/kgbwt of sodium pentobarbitone. Blood samples were then taken from each rat and put into a plain sample bottle. The blood samples were centrifuged at 2000 rpm for 10 min after being allowed to clot at room temperature for 2 h. With a fresh Pasteur pipette, the serum was separated. Prior to further biochemical analysis, serum samples were kept at − 80 °C. For liver enzyme analyses, the livers of the rats in each experimental group were removed, washed in normal saline, and stored in 10% formalin (v/v) [10].
Biochemical assays
Using different assay kits, the biochemical indices of the rats after 21-day of treatment with the extract and fractions to evaluate the level of high-density lipoprotein (HDL), cholesterol concentration Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total proteins, albumins, creatinine total cholesterol, and triglyceride. Additionally, the serum LDL-C concentration was determined according to the formula described by Kumari et al. [17] and previously reported by Oluba et al. [31].
Serum VLDL-C concentration was estimated using the methods of Burnstein and Sammaille [32] where the ratio of serum VLDL-C to triglyceride concentrations was fixed at 1:5 in fasting animals.
In vivo antioxidant enzyme activity
After sacrifice, the livers of the rats in each treatment group were immediately removed, washed with chilled saline solution, homogenized in ice-cold sucrose (10% w/v) and centrifuged at 10,000 g for 20 min. at 4 °C [33]. The activity of catalase (CAT), superoxide dismutase (SOD), and reduced glutathione (GSH) were assessed in the resulting supernatant.
Catalase activity
According to a method described by Luck (1974) and reported by Ahmad et al. [34], the activity of catalase (CAT) was assessed. 50 µL of the liver's supernatant was mixed with 2.5 mL of a buffer containing 30% H2O2 and vortexed for two minutes. After 30 s and subsequently 90 s, the reaction mixture's absorbance at 240 nm was measured. The blank contained only 2.5 mL of 30% H2O2 buffer. The CAT activity was measured using the formula as follows:
where ΔAbs is the difference in sample absorbance after 30 and 90 secs.
Superoxide dismutase activity
The activity of the superoxide dismutase (SOD) enzyme was measured using the method described by Misra and Fridovich [35]. The reaction mixture consists of 20 µL of liver homogenate, 960 mL of sodium carbonate buffer (50 mM, pH 10.2), and 0.1 mM EDTA. Furthermore, 20 µL of 30 mM epinephrine, dissolved in 0.05% v/v acetic acid, were added to the mixture to initiate the reaction. The blank and control contained sodium carbonate buffer and distilled water, respectively. The increase in absorbance was measured at 480 nm for 4 min and activity calculated as follows:
where ΔAbs control is the difference in control absorbance at different times and ΔAbs sample is the difference in control absorbance at different times.
Reduced glutathione concentration
Reduced GSH content was assayed following the method described by Jollow et al. [36]. The liver homogenate (50 μL) was mixed with 150 μL of sulfosalicylic acid (SSA) and centrifuged at 5000 × g for 10 min at 4 °C. The amount of GSH was determined by mixing 66 μL of supernatant with 66 μL of 0.01 M 5,5-dithiobis-2-nitrobenzoic acid (DTNB) and 865 μL of potassium phosphate buffer (0.1 M, pH 7.4). After 5 min, the absorbance was measured against SSA as blank at 412 nm and the concentration calculated as follows:
where Abs is the sample absorbance.
Statistical and data analyses
The data collected were analyzed using one-way analysis of variance (ANOVA) while treatment means was separated by the least significance difference (LSD) incorporated in the statistical package for social sciences (SPSS) version 20.
Results
Phytochemical constituents of N. canescens extract and fractions
The constituents of N. canescens extracts and fractions show a varying degree of phytochemicals present. Alkaloids (56.59 ± 1.40 mg/g) and tannins (76.21 ± 0.04 mg/g) were significantly higher in the ethylacetate fraction compared to the aqueous extract and methanol fraction. Phenols was significantly higher (p < 0.05) in the aqueous extract (49.65 ± 8.10 mg/g) compared to ethylacetate and methanol fractions while all the phytochemicals’ constituents were generally distributed in low abundance in the methanol fraction. Flavonoid content (20.60 ± 0.20 mg/g) of aqueous extract was higher than the fraction while methanol fraction has the highest amount of saponins (17.61 ± 0.26 mg/g).
In vitro antioxidant potentials of N. canescens extracts and fractions
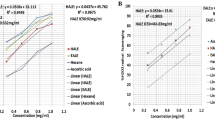
The antioxidant capacity of N. canescens aqueous extracts and EA and ME fractions was measured based on their ability of reducing ABTS and DPPH radicals. The results show that the radical scavenging capacity of N. canescens aqueous extract and fractions occurs in a dose dependent manner when compared to the standard ascorbic acid (Fig. 1). The IC50 values of the EA fraction (31.03 ± 0.89 µg/mL) and ME fraction (37.60 ± 1.20 µg/mL) were lower than that of the aqueous extract (50.10 ± 0.26 µg/mL) in the ABTS assay, with no significant changes observed relative to the standard ascorbic acid control standard (38.91 ± 0.25 µg/mL).
Similar to what was observed in the ABTS assay, the DPPH assay also shows a dose-dependent inhibition of the DPPH radicals by the N. canescens aqueous extract and fractions with no significant differences observed in the IC50 values of the same treatments relative to the standard ascorbic acid control (Fig. 1).
Bars with the same color and the same alphabet have no significance difference at p < 0.05.
Effect of N. canescens extracts on blood glucose levels of alloxan-induced diabetic rats
The antidiabetic properties of N. canescens extracts and fractions (EA and ME) in alloxan-induced diabetic rats were evaluated. The results show that the extract and respective fractions significantly reduced (p < 0.05) the blood glucose level in a dose-dependent manner. It is worth noting that the rats used in the diabetic control group did not survive beyond Day 12 of treatment due to a significant spike in blood glucose levels over a period of 6 days. Although a significant increase in glucose levels was observed in the Glibenclamide treatment for the first 3 days, this increase was gradually reduced over time and reached the levels of the normal saline control after 21 days.
Changes in blood glucose levels of alloxan-induced diabetic rats treated with N. canescens extracts and fractions
The aqueous extract of N. canescens reduced (p < 0.05) the blood glucose levels in alloxan-induced diabetic rats in a concentration dependent manner with the highest reduction of 74.35 ± 11.33 observed in the AQ 300 mg/kgbwt treatment group (Table 2). A similar phenomenon was observed in the EA and ME fraction treatment groups. The EA fraction (300 mg/kgbwt) reduced glucose levels by (81.86 ± 5.27%) whereas the ME fraction (300 mg/kgbwt) caused a reduction of 78.66 ± 9.42%.
Effect of N. canescens extract and fractions on the lipid profile (mg/dL) of alloxan-induced diabetic rats
The effect of N. canescens extract and fractions (EA and ME) on the lipid profile of alloxan-induced diabetic rats was in a dose-dependent manner across all the groups; however, rats treated with 300 mg/kgbwt aqueous extract have a significant reduction (p < 0.05) in the total cholesterol; HDL and LDL cholesterol were compared with the diabetic control and the normal control groups (Table 3).
Effects of N. canescens extract and fractions on biochemical parameters and serum enzymes in alloxan-induced diabetic rats
The aqueous extract and fractions (EA and ME) of N. canescens differentially altered biochemical parameters and serum enzyme activity in alloxan-induced diabetic rats when compared to the normal and diabetic controls (Table 4). Apart from treatment with EA (300 mg/kgbwt) and ME (50 mg/kgbwt and 300 mg/kgbwt) fractions of N. canescens, no significant changes in total protein (mg/dL) were observed when compared to the normal and diabetic controls.
For the detection of Albumin (mg/dL) in alloxan-induced diabetic rats, no significant change was observed in all treatments except for the ME fraction (50 mg/kgbwt). Ethylacetate fraction at 50 and 300 mg/kgbwt significantly increased the creatinine level of the rats compared with the Glibenclamide treated groups. In the aqueous extract of N. canescens, respectively, there was an increase in the ALT levels in the animal’s serum compared with the control groups. In addition, the aqueous extract (50 and 300 mg/kgbwt), EA fraction (300 mg/kgbwt) and ME fractions (50 and 300 mg/kgbwt) of N. canescens significantly increase the AST level of rats relative to the normal and diabetic controls (Table 4).
Effects of N. canescens extracts and fractions on liver antioxidant enzyme activities in alloxan-induced diabetic rats
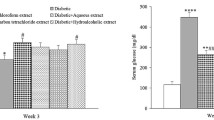
The hepatoprotective effects of N. canescens extract and fractions (EA and ME) were evaluated by monitoring changes in liver antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione (GSH) as shown in Table 5.
The results show a dose-dependent regulation of antioxidant activity by N. canescens extract and fractions (EA and ME) relative to the Glibenclamide (5 mg/kgbwt of) and diabetic control groups. CAT activity for all treatments was significantly lower than that observed in the diabetic control (274.10 ± 16.76) and Glibenclamide treatment group (235.38 ± 27.66), respectively.
SOD activity in all treatments was significantly higher than observed in the normal saline control. The highest increase in SOD activity (75.09 ± 0.00) was observed in the ME (50 mg/kgbwt) treatment group relative to the diabetic and saline controls.
For GSH activity, no significant changes were observed in the ME fraction treatment groups (50 mg/kgbwt and 300 mg/kgbwt) when compared to the saline control. Both doses of the N. canescens extract (50 mg/kgbwt and 300 mg/kgbwt) showed an increase in GSH activity to levels higher than observed for the saline and diabetic control groups with the highest increase (103.31 ± 6.14) observed in the 50 mg/kgbwt treatment. A similar trend was observed in the EA fraction treatment group.
Discussion
The present study evaluates the antidiabetic activity of the aqueous extract of Nelsonia canescens and its partitioned fractions (EA and ME) in alloxan-induced diabetic rats. Quantitative phytochemical analysis of the extract and fractions revealed the presence of phenols, flavonoids, tannins, alkaloids and saponins at varying concentrations (Table 1). These results agreed with earlier reports by Daniel et al. [23] Haruna et al. [24] who showed the presence of these phytochemicals in methanol and chloroform extracts of N. canescens. The bioactivity or medicinal potentials of any plant is dependent on the presence and abundance of these phytochemicals [37]. Phenols, flavonoids, saponins, tannins and alkaloids possess good antidiabetic [37], anti-inflammatory [38], antibacterial [13], antiseptic and antioxidant [26], hemostatic, hypocholesterolaemic and anticarcinogenic activities [39]. Hence, the presence of these phytochemicals in the extract maybe attributed to its pharmacological importance of the plant as demonstrated in this study.
A major characteristic feature of a diabetic condition is the generation of free radicals in diabetic patients. Therefore, it is important to search for therapeutics that will not only manage the disease but also place emphasis on other complications associated with the disease. In vitro antioxidant activity of the aqueous extract of N. canescens and its fractions using DPPH and ABTS models (Fig. 1) shows a dose-dependent inhibition of the radicals in both assays. The median inhibition concentration (IC50) which is the concentration required to inhibit 50% of the radicals is used to classify the antioxidant activities of chemicals as either very strong (< 50 µg/mL), strong (50–100 µg/mL) and weak (100–150 µg/mL) antioxidants [39, 40]. Based on this classification, the IC50 values of the aqueous extract and fractions of N. canescens are regarded as very active antioxidants except in the ABTS assay where the aqueous extract produced an IC50 value of 50.10 ± 0.26 µg/mL and is therefore classified as a strong antioxidant (Fig. 1). ABTS and DPPH are reliable models to evaluate the antioxidant potential of chemicals and natural products by arresting their radicals in solution [41]. The observed antioxidant properties demonstrated by the extract may also be attributed to phytochemicals such as phenols, flavonoids and tannins present in an appreciable amount in the extract which are natural antioxidants that exhibit their antioxidant potentials by donating the proton of their hydroxyl group hence arresting the free radicals in the system [42].
The use of alloxan monohydrate for the induction of type-2 diabetes is well documented [13]. Alloxan induces diabetes by altering the function of the β-cells of the islets of Langerhans in the pancreas leading to a decreased endogenous insulin thus affecting the ability of the tissue to utilize glucose [13, 43]. Antidiabetic activity of the extract and fractions shows a significant (p < 0.05) dose-dependent reduction of the blood glucose of rats which are significantly different to the diabetic control group (Fig. 2). The ethylacetate fraction at 300 mg/kgbwt showed the highest blood glucose reduction (81.86 ± 5.27%) followed by the 300 mg/kgbwt of the methanol fraction and the aqueous extract with a percentage reduction of 78.66 ± 9.42 and 74.35 ± 11.33%, respectively (Table 2). The observed level of activity in the extract and fractions may be attributed to the phytochemical constituents of the plants such as phenols, flavonoids, tannins and saponins which can mimic insulin or excite the secretion of β-cells by the islets of Langerhans [43, 44]. The antidiabetic properties of phenols and flavonoids are attributed to their ability to change the process of glucose and oxidative metabolisms in a diabetic condition and also by exerting a stimulatory effect on insulin secretion altering Ca2+ concentration [25, 38]. Also, flavonoids have been reported to modulate blood glucose levels and could regulate key rate-determining enzymes such as hexokinase, phosphofructokinase-1 and pyruvate kinase which are vital for metabolic pathways of carbohydrates [12]. Alkaloids, glycosides and saponins have similarly been implicated in the antidiabetic activities of plants [13].
In diabetic state, elevated blood glucose is often accompanied with dyslipidemia denoting risk factor for atherosclerosis [25, 45, 46]. Elevated levels of serum lipids are largely due to the uninhibited actions of lipolytic hormones on the adipose tissue as a result of insulin inactivity [25, 46]. Insulin is responsible for the activation of lipoprotein lipase an enzyme which hydrolyzes triglycerides (TGs) under normal circumstances [45]. Nevertheless, lipoprotein lipase is not activated in diabetic state because of insulin deficiency, leading to hypertriglyceridemia and hypercholesterolemia due to metabolic abnormalities [47]. TGs excite the secretion of very low-density lipoprotein cholesterol (VLDL); hence, increase in very low-density lipoprotein cholesterol particles reduces the high-density lipoprotein cholesterol (HDL-C) level and increases the low-density lipoprotein (LDL-C) particles [48]. A major feature of diabetic dyslipidemia is elevated level of serum triglycerides, total cholesterol, LDL-Cholesterol, and a decrease in HDL-Cholesterol levels [25, 49]. An altered serum lipid profile was observed in diabetic control group compared to the groups treated with extract and fractions of N. canescens (Table 3). The alteration in serum lipid profile however was reversed toward normal levels in the extract and fractions treated groups; hence, the extract and fractions may be useful as cardio-protective agent in enhancing the breakdown of lipid which may subsequently help to prevent diabetic complications such as coronary heart diseases and atherosclerosis [12].
Creatinine, total proteins, albumin, ALT and AST levels are used as biochemical markers of liver and kidney injuries; thus, elevated levels of these markers may indicate liver or kidney dysfunction [50]. The result presented here shows a significant increase (p < 0.05) in creatinine levels in non-dose dependent manner when compared to the diabetic control (Table 4). Creatinine is a non-protein nitrogenous substance formed from creatine and phosphocreatine during muscle metabolism and excreted through glomerular filtration. The rate of excretion of creatinine is influenced by the glomerular filtration rate (GFR), so any abnormality that decreases GFR will result in increased serum creatinine [51]. The significant increase (p < 0.05) in creatinine levels in the groups treated with 50 and 300 mg/kgbwt of the EA fraction may be an indication of kidney impairments due to the adverse effect of the treatment [52]. No significant difference (p > 0.05) in albumin content was observed between the treatments relative to the control groups. Total protein content was significantly increased (p < 0.05) in both EA and ME fractions (50 and 300 mg/kgbwt) when compared to the diabetic control group which suggest that these fractions may possess some hepatoprotective properties because albumin and total proteins are also used to determine a dysfunction in liver integrity [53].
The first line of defense against reactive oxygen and nitrogen species (ROS and RNS) induce damage in a living system is enzymatic and nonenzymatic antioxidants [54]. Three major scavenging antioxidants responsible for the removal of toxic free radicals in vivo are superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) [45]. By accelerating the elimination of superoxide radical and converting it to H2O2 and molecular oxygen, which both harm the cell membrane and other biological components, SOD protect tissues against free radicals [45]. A hemeprotein called CAT oversees detoxifying good amount of H2O2 in the body [55]. GSH catalyzes the detoxification of endogenous metabolic peroxides and hydroperoxides as well as the catabolism of H2O2 [56]. A crucial co-substrate for GSH-Px, glutathione serves as a free radical scavenger [45]. In comparison to the diabetic control group, the activity of these enzymes was substantially different (p < 0.05) in the extract and fractions treated groups and the Glibenclamide (reference group) (Table 5). This serves to further support the extract's safety as reported by Haruna et al. [24], who found that the extract's LD50 was above 2000 mg/kgbwt and that it may help treat diabetes and other health related conditions.
Conclusion
In conclusion, the outcome of this study shows that the extract and respective fractions (EA and ME) of N. canescens have hypoglycemic and hypolipidemic potentials to effectively reduce the progression of oxidative stress in alloxan-induced diabetic rats.
Availability of data and materials
Data will be available by corresponding author upon reasonable request.
Abbreviations
- ABTS:
-
2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
- ALT:
-
Alanine aminotransferase
- AQ:
-
Aqueous extract
- AST:
-
Aspartate aminotransferase
- DM:
-
Diabetes mellitus
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- EA:
-
Ethylacetate fraction
- EDTA:
-
Ethylenediamine tetraacetate
- FC:
-
Folin–Ciocalteu
- FPGC:
-
Fasting plasma glucose concentration
- GDM:
-
Gestational diabetes mellitus
- HDL-C:
-
High density lipoprotein cholesterol
- IDDM:
-
Insulin dependent diabetes mellitus
- LDL-C:
-
Low density lipoprotein cholesterol
- ME:
-
Methanol fraction
- NIDDM:
-
Non-insulin dependent diabetes mellitus
- PBS:
-
Phosphate buffer saline
- SOD:
-
Superoxide dismutase
- TG:
-
Triglycerides
References
Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, Borodo MM, Sada KB (2018) Prevalence and risk factors for diabetes mellitus in Nigeria: a systematic review and meta-analysis. Diabetes Ther 9(3):1307–1316
Verma S, Gupta M, Popli H, Aggarwal G (2018) Diabetes mellitus treatment using herbal drugs. Int J Phytomedicine 10(1):1
Pottathil S, Nain P, Morsy MA, Kaur J, Al-Dhubiab BE, Jaiswal S, Nair AB (2020) Mechanisms of antidiabetic activity of methanolic extract of Punica granatum leaves in nicotinamide/streptozotocin-induced type 2 diabetes in rats. Plants 9(11):1609
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843
Wannes WA, Marzouk B (2016) Research progress of Tunisian medicinal plants used for acute diabetes. J Acute Dis 5(5):357–363
Chawla A, Chawla R, Jaggi S (2016) Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab 20(4):546
David S, Paul Z (2011) Diabetes and hyperlipidemia: a direct quantitative analysis—a direct analysis of the effects of insulin resistance on lipid levels in relation to atherosclerotic coronary artery disease. World J Cardiol 31:2012
Basak RC, Chatterjee M, Sarma PS (2013) An overview on management of diabetic dyslipidemia. J Diabetes Endocrinol 4(3):27–36
Chahil TJ, Ginsberg HN (2006) Diabetic dyslipidemia. Endocrinol Metab Clin North Am 35(3):491–510
Belayneh YM, Birru EM, Ambikar D (2019) Evaluation of hypoglycemic, antihyperglycemic and antihyperlipidemic activities of 80% methanolic seed extract of Calpurnia aurea (Ait.) Benth. (Fabaceae) in mice. J Exp Pharmacol 11:73
Ogbonna JI, Umeh ON, Mbah CC, Ofoefule SI, Ozumba BC (2019) Hypoglycemic activities and biochemical parameters modulation of herbal formulations of Allium cepa L. in alloxanized diabetic rats. Sci Res Essays 14(10):74–85
Sudasinghe HP, Peiris DC (2018) Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. PeerJ 6:e4389
Akah PA, Uzodinma SU, Okolo CE (2011) Antidiabetic activity of aqueous and methanol extract and fractions of Gongronema latifolium (Asclepidaceae) leaves in alloxan diabetic rats. J Appl Pharm Sci 1(9):99
Malviya N, Jain S, Malviya SA (2010) Antidiabetic potential of medicinal plants. Acta Pol Pharm 67(2):113–118
Villas Boas GR, Rodrigues Lemos JM, de Oliveira MW, dos Santos RC, Stefanello da Silveira AP, Barbieri Bacha F, Ito CN, Bortolotte Cornelius E, Brioli Lima F, Sachilarid Rodrigues AM, Belmal Costa N (2020) Aqueous extract from Mangifera indica Linn. (Anacardiaceae) leaves exerts long-term hypoglycemic effect, increases insulin sensitivity and plasma insulin levels on diabetic Wistar rats. PLoS ONE 15(1):e0227105
Arya A, Abdullah MA, Haerian BS, Mohd MA (2012) Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: in-vivo evaluation. E-J Chem 9(3):1196–1205
Kumari MS, Lakshmi KN, Prasanna TV, Swapna K, Jyothi AS, Prasanthi T (2016) Natural herbs vs allopathic drugs: to treat diabetes. Indo Am J Pharm 3(5):415–422
Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S (2017) Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag 20(1):6–12
Narayan DS, Patra VJ, Dinda SC (2012) Diabetes and indian traditional medicines an overview. Int J Pharm Pharm Sci 4(3):45–53
Prabhakar PK, Doble M (2011) Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med 17(8):563–574
Acharya RN, Padiya RH, Patel ED, Harisha CR, Shukla VJ, Chauhan MG (2012) Pharmacognostic evaluation of Nelsonia canescens. (Lam) Spreng. (Acanthaceae) Root. Pharmacogn. J. 4(28):45–48
Mohaddesi B, Ashok BK, Acharya R, Shukla VJ (2015) Anti-inflammatory activity of Nelsonia canescens. (Lam) Spreng. root in albino rats. J Phytopharm 4(2):68–72
Daniel AI, Gara TY, Ibrahim YO, Mohammad FM, Salisu FE, Tsado R, Agboola AM (2022) In vivo antidiabetic and antioxidant activities of chloroform fraction of Nelsonia canescens Leaf in Alloxan-induced Diabetic Rats. Pharmacol Res-Modern Chin Med. 3:100106
Haruna Y, Isah I, Elinge CM (2018) Median lethal dose and phytochemical studies of aqueous leaves extract of blue pussy leaf Nelsonia canescens (Lam.) Spreng. Ann Clin Toxicol 1(1):1010
Elberry AA, Harraz FM, Ghareib SA, Gabr SA, Nagy AA, Abdel-Sattar E (2015) Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int J Diabetes Mellit 3(1):37–44
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. https://doi.org/10.38212/2224-6614.2748
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol 299:152–178
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Gyamfi MA, Yonamine M, Aniya Y (1999) Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. General Pharmacol Vasc Syst 32(6):661–667
Chikezie PC (2015) Histopathological studies of renal and hepatic tissues of hyperglycemic rats administered with traditional herbal formulations. Int J Green Pharm 9(3):184–191
Oluba OM, Olusola AO, Eidangbe GO, Babatola LJ, Onyeneke EC (2012) Modulation of lipoprotein cholesterol levels in Plasmodium berghei malarial infection by crude aqueous extract of Ganoderma lucidum. Cholesterol 2012:2012
Burnstein MA, Sammaille J (1960) A rapid determination of cholesterol bound to A and B-lipoprotein. Clin Chem Acta 5:601–609
Awad S, El-Sayed MI, Wahba A, El Attar A, Yousef MI, Zedan M (2016) Antioxidant activity of milk protein hydrolysate in alloxan-induced diabetic rats. J Dairy Sci 99(11):8499–8510
Ahmad P, Abdel Latef AA, Hashem A, Abd Allah EF, Gucel S, Tran LS (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. JBC 247(10):3170–3175
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169
Daniel AI, Ewansiha JU, Amuda OA, Vivian N (2016) Phytochemical and antimicrobial study of seventy percent methanol leaf extract of Terminalia microptera against selected pathogenic bacteria. JAMPS 9(1):1–8
Innalegwu DA, Uyi EJ, Emmanuel MB, Ufedo OQ. of Terminalia microptera Leaf. Quantitative Phytochemical and Antibacterial Efficacy of Fractions of Terminalia microptera Leaf. Am J Pharm Pharmacol 4(5):35–40
Marjoni MR, Zulfisa A (2017) Antioxidant activity of methanol extract/fractions of senggani leaves (Melastoma candidum D. Don). Pharm Anal Acta 8(8):1–6
Simorangkir M, Nainggolan B, Silaban S (2019) Antioxidant activity of vacuum column chromatography fractions of ethanol extract of sarang banua (Clerodenrum fragrans vent willd) leaves. In J Phys Conf Ser 1374(1):012016
Wani NA, Tirumale S (2018) Evaluation of antioxidant properties of different extracts of Chaetomium cupreum SS02. Bull Fac Pharm Cairo Univ 56(2):191–198
Mahdi-Pour B, Jothy SL, Latha LY, Chen Y, Sasidharan S (2012) Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac J Trop Biomed 2(12):960–965
Miaffo D, Guessom Kamgue O, Ledang Tebou N, Maa Maa Temhoul C, Kamanyi A (2019) Antidiabetic and antioxidant potentials of Vitellaria paradoxa barks in alloxan-induced diabetic rats. Clin Phytoscience 5(1):1–8
Ikewuchi JC, Onyeike EN, Uwakwe AA, Ikewuchi CC (2011) Effect of aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’Muell Arg (Euphorbiaceae) on the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan induced diabetic rats. J Ethnopharmacol 37(3):1415–1424
Hassan SK, El-Sammad NM, Mousa AM, Mohammed MH, Hashim AN, Werner V, Lindequist U, Nawwar MA (2015) Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 5(6):462–471
Kumar R, Arora V, Ram V, Bhandari A, Vyas P (2015) Hypoglycemic and hypolipidemic effect of Allopolyherbal formulations in streptozotocin induced diabetes mellitus in rats. Int J Diabetes Mellit 3(1):45–50
Girija K, Lakshman K, Udaya C, Sachi GS, Divya T (2011) Anti–diabetic and anti–cholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pac J Trop Biomed 1(2):133–138
Singh SU, Garg VE, Yadav DE (2013) Antihyperglycemic and antioxidative ability of Stevia rebaudiana (Bertoni) leaves in diabetes induced mice. Int J Pharm Pharm 5(2):297–302
Karim MN, Ahmed KR, Bukht MS, Akter J, Chowdhury HA, Hossain S, Anwar N, Selim S, Chowdhury SH, Hossain F, Ali L (2013) Pattern and predictors of dyslipidemia in patients with type 2 diabetes mellitus. Diabetes Metab Syndr 7(2):95–100
Rajakrishnan R, Lekshmi R, Benil PB, Thomas J, AlFarhan AH, Rakesh V, Khalaf S (2017) Phytochemical evaluation of roots of Plumbago zeylanica L. and assessment of its potential as a nephroprotective agent. Saudi J Biol Sci 24(4):760–766
Konda VR, Arunachalam R, Eerike M, Radhakrishnan AK, Raghuraman LP, Meti V, Devi S (2016) Nephroprotective effect of ethanolic extract of Azima tetracantha root in glycerol induced acute renal failure in Wistar albino rats. J Tradit Complement Med 6(4):347–354
Ogbe RJ, Agbese SP, Abu AH (2020) Protective effect of aqueous extract of Lophira lanceolata leaf against cisplatin-induced hepatorenal injuries and dyslipidemia in Wistar rats. Clin Phytoscience 6(1):1–1
Ukpabi SE, Offor CE, Udeozor PA, Obiudu I (2018) The Effects of Ethanol Leaf-Extract of Albizia ferruginea on some Liver and Histopathological Parameters in Wistar Albino Rats. Int J Sci Res Innov 3(2):60–70
Sellamuthu PS, Arulselvan P, Kamalraj S, Fakurazi S, Kandasamy M (2013) Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. Int Sch Res Notices 2013. https://doi.org/10.1155/2013/750109
Al-Shiekh AA, Al-Shati AA, Sarhan MA (2014) Effect of white tea extract on antioxidant enzyme activities of Streptozotocin–induced diabetic rats. Egypt Acad J Biol Sci 6(2):17–30
Saravanan G, Ponmurugan P (2013) S-allylcysteine improves streptozotocin-induced alterations of blood glucose, liver cytochrome P450 2E1, plasma antioxidant system, and adipocytes hormones in diabetic rats. Int J Endocrinol Metab. https://doi.org/10.5812/ijem.10927
Acknowledgements
The authors are grateful to the staff and managements of the Centre for Genetic Engineering and Biotechnology, Federal University of Technology Minna for providing the facility and enabling environment needed to carry out this research.
Funding
The present research work was privately funded by all authors.
Author information
Authors and Affiliations
Contributions
The study was conceptualized by AID and AK, and methodology was designed by AID, TYG, SOA, AMA and RT. Samples collections were carried out by SOA, AMA and RT and FES. The experiments were carried out by AID, AID, TYG, SOA, and A. Statistical analysis and validation of results were carried out by AID, MK, AOF and AK. Draft of the original manuscript was carried out by AID, TYG, SOA, RT, AMA, AOF, VM, MK and AK. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the use of animal was obtained from the Research and Ethical Committee of Federal University of Technology Minna, Niger State, Nigeria and assigned a number: 000018EAU.
Consent for publication
Not applicable.
Competing interests
There is no competing interest for this study.
Study involving plants
The plant sample was collected from the environment of Federal University of Technology Minna, Niger State. Plant identification was authenticated by Dr. Mohammed Dangana of Plant Biology Department, Federal University of Technology Minna. Permissions and/or licenses for the study were not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Daniel, A.I., Gara, T.Y., Atolagbe, S.O. et al. Nelsonia canescens (Acanthaceae) aqueous extract and partitioned fractions ameliorates type-2 diabetes in alloxan-induced diabetic rats. Futur J Pharm Sci 8, 53 (2022). https://doi.org/10.1186/s43094-022-00445-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-022-00445-1