Abstract
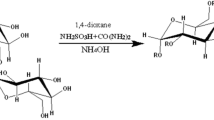
The “green” synthesis of galactomannan sulfates using a sulfamic acid–urea mixture has been studied for the first time. The effect of the time and temperature of the galactomannan sulfation process on the degree of substitution of galactomannan sulfates has been investigated. It is shown that, at a temperature of 70 °C with an increase in the process time up to 120 min, the degree of substitution increases up to 0.70. An increase in the process temperature up to 80 °C leads to the production of galactomannan sulfates with a degree of substitution of 1.67. With a further increase in the process temperature to 90 °C, the galactomannan structure is partially destructed, and the degree of substitution decreases. Embedding of the sulfate groups into the galactomannan structure has been confirmed by elemental analysis and Fourier-transform infrared spectroscopy. In addition, the initial and sulfated galactomannans have been characterized by X-ray diffraction, scanning electron microscopy, atomic force microscopy, and gel permeation chromatography. The thermal analysis shows that the initial galactomannan exhibits endothermic peaks at 254 and 294 °C and an exothermic peak at 315 °C, while sulfated galactomannan exhibits endothermic peaks at 209 and 275 °C and an exothermic peak at 281 °C. Using atomic force microscopy, it has been shown that the sulfated galactomannan film consists of spherical particles with an average diameter of 200–300 nm; according to the phase contrast data, it has the uniform composition without extraneous impurities.









Similar content being viewed by others
References
Dorea CMPG, Alvesa MGCF, Willa LSEP, Costa TG, Sabry DA (2012) A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr Polym 91:467–475. https://doi.org/10.1016/j.carbpol.2012.07.075
Oliveira RCR, Almeida RR, Goncalves TA (2016) A review of plant sulfated polysaccharides and their relations with anticoagulant activities. J Dev Drugs 5:3. https://doi.org/10.4172/2329-6631.1000166
Kaith BS, Sharma R, Kalia S (2015) Guar gum based biodegradable, antibacterial and electrically conductive hydrogels. Int J Biol Macromol 75:266–275. https://doi.org/10.1016/j.ijbiomac.2015.01.046
Thombare N, Jha U, Mishra S, Siddiqui MZ (2016) Guar gum as a promising starting material for diverse applications: a review. Int J Biol Macromol 88:361–372. https://doi.org/10.1016/j.ijbiomac.2016.04.001
Da Silva BP, Parente JP (2002) Chemical properties and biological activity of a polysaccharide from Melocactus depressus. Planta Med 68(1):74–76. https://doi.org/10.1055/s-2002-20052
Gracher AHP, Santana AG, Cipriani TR, Lacomini M (2015) A procoagulant chemically sulfated mannan. Carbohydr Polym 136:177–186. https://doi.org/10.1016/j.carbpol.2015.09.022
Reis RL (2008) Natural-based polymers for biomedical applications / Reis R L, Neves N M , Mano J F, Gomes M E, Marques A P, Azevedo H S. – Lisboa: Elsevier 832 p
Goun EA, Petrichenko VM, Solodnikov SU, Suhinina TV (2002) Anti cancer and anti-thrombin activity of Russian plants. J Ethnopharmacol 81:337–342. https://doi.org/10.1016/s0378-8741(02)00116-2
Silveira JLM, Bresolin TMB (2011) Pharmaceutical use of galactomannans. Quim Nova 34(2):292–299
Cerqueira MA, Bourbon AI, Pinheiro AC, Martins JT, Souza BWS, Teixeira JA, Vicenta AA (2011) Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci Technol 22:662–671. https://doi.org/10.1016/j.tifs.2011.07.002
Prajapati VD, Jani GK, Moradiya NG, Randeria NP, Nagar BJ, Naikwadi NN, Variya BC (2013) Galactomannan: a versatile biodegradable seed polysaccharide. Int J Biol Macromol 60:83–92. https://doi.org/10.1016/j.ijbiomac.2013.05.017
Filatova AV, Azimova LB, Turaev AS (2020) Study of the process of gelation of galactomannan from the seeds of Styphnolobium japonicum (Fabaceae). Chem Plant Raw Mater 1:33–39. https://doi.org/10.14258/jcprm.2020015485
Mercier T, Guldentops E, Lagrou K, Maertens J (2018) Galactomannan, a surrogate marker for outcome in invasive Aspergillosis: finally coming of age. Front Microbiol 9:661. https://doi.org/10.3389/fmicb.2018.00661
Perera N, Yang FL, Chang CM, Lu YT, Zhan SH, Tsai YT, Hsieh JF, Li LH, Hua KF, Wu SH (2017) Galactomannan from Antrodia cinnamomea enhances the phagocytic activity of macrophages. Org Lett 19(13):3486–3489. https://doi.org/10.1021/acs.orglett.7b01468
Tolstenkov AC, Drozd HH, Lapikova EU, Makarov VA, Mestechkina NM, Bannikova GE, Ilyina AB, Varlamov VP (2007) Effect of galactomannan from seeds of Cyamopsis Tetragonoloba (L.) taub on anticoagulant activity of rat plasma with intravenous administration. Clin Hematol Hemorheol Cardiovasc Surg 7:242–243 (in Rus)
Mestechkina NM, Anulov OV, Scherbukhin VD (1998) Study of galactomannan seed Amorphafruticosa L. Appl Biochem Microbiol (Rus) 34(5):549–552
Mestechkina NM, Dovletmuradov K, Scherbukhin VD (1991) Galactomannan common licorice seeds (Glyzyrrhizaglabra). Appl Biochem Microbiol (Rus) 27(3):435–441
Krishtanova NA, Safonova MJ, Bolotova VTs (2005) Prospects for the use of plant polysaccharides as therapeutic and therapeutic agents. Proceed Voronezh St Univ Ser: Chem Biol Pharm 1:212–221. (in Russ)
Caputo HE, Strau JE, Grinstaff MW (2019) Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem Soc Rev 48:2338–2365. https://doi.org/10.1039/c7cs00593h
Vasilyeva NY, Levdanskiy AV, Kazachenko AS, Djakovich L, Pinel K, Kuznetsov BN (2013) Sulfation of mechanoactivated arabinogalactan with sulfuric anhydride–pyridine complex in pyridine medium. J Sib Fed Univ Chem 6(2):158–169
Mestechkina NM, Egorov AV, Shcherbukhin VD (2006) Synthesis of galactomannan sulfates. Appl Biochem Microbiol 42:326–330. https://doi.org/10.1134/S0003683806030185
Wang X, Wang J, Zhang J, Zhao B, Yao J, Wang Y (2009) Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int J Biol Macromol 46(1):59–66. https://doi.org/10.1016/j.ijbiomac.2009.10.004
Zhang Z, Wang H, Chen T, Zhang H, Liang J, Kong W, Yao J, Zhang J, Wang J (2019) Synthesis and structure characterization of sulfated galactomannan from fenugreek gum. Int J Biol Macromol 125:1184–1191. https://doi.org/10.1016/j.ijbiomac.2018.09.113
Kuznetsov BN, Levdansky VA, Kuznetsova SA, Garyntseva NV, Sudakova IG, Levdansky AV (2018) Integration of peroxide delignification and sulfamic acid sulfation methods for obtaining cellulose sulfates from aspen wood. Eur J Wood Prod 76(3):999–1007. https://doi.org/10.1007/s00107-017-1262-z
Al-Horani RA, Desai UR (2010) Chemical sulfation of small molecules – advances and challenges. Tetrahedron. 66(16):2907–2918. https://doi.org/10.1016/j.tet.2010.02.015
Wang J, Zhao B, Wang X, Yao J, Zhang J (2012) Structure and antioxidant activities of sulfated guar gum: homogeneous reaction using DMAP/DCC catalyst. Int J Biol Macromol 50(5):1201–1206. https://doi.org/10.1016/j.ijbiomac.2012.03.009
Sirvio JA, Ukkola J, Liimatainen H (2019) Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 26(4):2303–2316. https://doi.org/10.1007/s10570-019-02257-8
Akman F, Kazachenko AS, Vasilyeva NY, Malyar YN (2020) Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. J Mol Struct 1208:127899. https://doi.org/10.1016/j.molstruc.2020.127899
Park S, Baker JO, Himmel ME, Parilla PA, Jonson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on integrating cellulose performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Spillane W, Malaubier JB (2014) Sulfamic acid and its n- and o-substituted derivatives chemical reviews 114(4):2507–2586. https://doi.org/10.1021/cr400230c
Kuznetsov BN, Vasilyeva NY, Kazachenko AS, Skvortsova GP, Levdansky VA, Lutoshkin MA (2018) Development of the method of Abies wood ethanol lignin sulfation using sulfamic acid. J Sib Fed Univ Chem 1(11):122–130. https://doi.org/10.17516/1998-2836-0063
Muschin T, Yoshida T (2012) Structural analysis of galactomannans by NMR spectroscopy. Carbohydr Polym 87(3):1893–1898. https://doi.org/10.1016/j.carbpol.2011.08.059
Muschin T, Budragchaa D, Kanamoto T, Nakashima H, Ichiyama K, Yamamoto N, Shuqin H, Yoshida T (2016) Chemically sulfated natural galactomannans with specific antiviral and anticoagulant activities. Int J Biol Macromol 89:415–420. https://doi.org/10.1016/j.ijbiomac.2016.05.005
Wang J, Niu S, Zhao B, Wang X, Yao J, Zhang J, Zhao W, Zhao Y (2013) Regioselective synthesis of sulfated guar gum: comparative studies of structure and antioxidant activities. Int J Biol Macromol 62:734–740. https://doi.org/10.1016/j.ijbiomac.2013.10.005
Yin C, Shen X (2007) Synthesis of cellulose carbamate by supercritical CO2-assisted impregnation: structure and rheological properties. Eur Polym J 43:2111–2116. https://doi.org/10.1016/j.eurpolymj.2007.01.041
Heinze U, Klemm D, Unger E, Pieschel F (2003) New starch phosphate carbamides of high swelling ability: synthesis and characterization. Starch-Staerke 55:55–60. https://doi.org/10.1002/star.200390017
Willberg-Keyrilainen P, Hiltunen J, Ropponen J (2018) Production of cellulose carbamate using urea-based deep eutectic solvents. Cellulose 25:195–204. https://doi.org/10.1007/s10570-017-1465-9
Mudgil D, Barak S, Khatkar BS (2012) X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int J Biol Macromol 50(4):1035–1039. https://doi.org/10.1016/j.ijbiomac.2012.02.031
Bhushan B, Fuchs H, Hosaka S (2004) Applied scanning probe methods I. Springer-Verlag, New York
Kuznetsov BN, Vasilyeva NY, Levdansky AV, Karacharov AA, Krylov AS, Mazurova EV, Bondarenko GN, Levdansky VA, Kazachenko AS (2017) The Raman spectroscopy, XRD, SEM, and AFM study of Arabinogalactan sulfates obtained using sulfamic acid. Rus J Bioorg Chem 43(7):722–726. https://doi.org/10.1134/S106816201707010X
Cerqueira MA, Souza BWS, Simões J, Teixeira JA, Domingues MRM, Coimbra MA, Vicente AA (2011) Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr Polym 83(1):179–185. https://doi.org/10.1016/j.carbpol.2010.07.036
Ramos-Sanchez MC, Rey FJ, Rodriguez ML, Martin-Gil FJ, Martin-Gil J (1988) DTG and DTA studies on fungical polysaccharides. Thermochim Acta 134:55–60. https://doi.org/10.1016/0040-6031(88)85217-1
Rey FJ, Ramos-Sanchez MC, Rodriguez-Mendez ML, Martin-Gil J, Martin-Gil FJ (1988) DTG and DTA studies on typical sugars. Thermochim Acta 134:67–72. https://doi.org/10.1016/0040-6031(88)85216-X
Zohuriaan MJ, Shokrolahi F (2004) Thermal studies on natural and modified gums. Polym Test 23:575–579. https://doi.org/10.1016/j.polymertesting.2003.11.001
Chaires-Martínez L, Salazar-Montoya JA, Ramos-Ramírez EG (2008) Physicochemical and functional characterization of the galactomannan obtained from mesquite seeds (Prosopis pallida). Eur Food Res Technol 227:1669–1676. https://doi.org/10.1007/s00217-008-0892-0
Vendruscolo CW, Ferrero C, Pineda EAG, Silveira JLM, Freitas RA, Jiménez-Castellanos MR, Bresolin TMB (2009) Physicochemical and mechanical characterization of galactomannan from Mimosa scabrella: effect of drying method. Carbohydr Polym 76(1):86–93. https://doi.org/10.1016/j.carbpol.2008.09.028
Wang Q, Ellisa PR, Ross-Murphy SB (2003) Dissolution kinetics of guar gum powders—II. Effects of concentration and molecular weight. Carbohydr Polym 53:75–83. https://doi.org/10.1016/S0144-8617(03)00009-2
Hirata T, Nishimoto T (1991) DSC, DTA, and TG of cellulose untreated and treated with flame-retardants. Thermochim Acta 193:99–106. https://doi.org/10.1016/0040-6031(91)80177-K
Varma AJ, Kokane SP, Pathak G, Pradhan SD (1997) Thermal behavior of galactomannan guar gum and its periodate oxidation products. Carbohydr Polym 32(2):111–114. https://doi.org/10.1016/S0144-8617(96)00155-5
Acknowledgments
The experiments were conducted on the equipment of the Krasnoyarsk Territorial Center for Collective Use, Federal Research Center «Krasnoyarsk Science Center SB RAS».
Funding
The reported study was funded by RFBR, project number 20-33-70256.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazachenko, A.S., Malyar, Y.N., Vasilyeva, N.Y. et al. «Green» synthesis and characterization of galactomannan sulfates obtained using sulfamic acid. Biomass Conv. Bioref. 12, 2705–2714 (2022). https://doi.org/10.1007/s13399-020-00855-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00855-2