Abstract
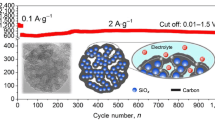
SiO2-based anodes have attracted extensive attention due to their high theoretical capacity of 1,956 mAh g-1, stable cycle life, and abundance on Earth. However, their commercialization is still hindered by several intrinsic problems, such as poor electrical conductivity and electrochemical inactiveness. In this study, a 3-dimensional SiO2/C electrode is fabricated by introducing a pore-forming agent (polytetrafluoroethylene, PTFE) and partially carbonizing a polyvinylidene fluoride (PVDF) binder. During heat treatment at 600 °C, PTFE powders are unzipped to develop microsized pores. Meanwhile, the PVDF binder is partially carbonized to form highly conductive F-doped graphitic carbon. In particular, a highly porous platelet SBA-15 template is used as an SiO2 active material for large contact areas between SiO2 and carbonized PVDF. As a result, the structured SiO2/C anode exhibits better cycle performance and internal resistance than typical SiO2 electrodes: the structured SiO2/C anode delivers 294 mAh g-1, while the typical SiO2 anode is electrochemically inactive with Li+ ions.
Graphical Abstract










Similar content being viewed by others
Change history
22 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13391-022-00335-x
References
Armand, M., Tarascon, J.M.: Building better batteries. Nature 451, 652 (2008)
Yao, Y., et al.: A carbon mixed amorphous-TiSx separator coating for lithium sulfur batteries. Mater. Chem. Phys. 258, 123923 (2021)
Chung, W.Y., et al.: Petroleum waste hydrocarbon resin as a carbon source modified on a Si composite as a superior anode material in lithium ion batteries. Mater. Chem. Phys. 259, 124011 (2021)
Zhang, L., et al.: Synthesis of N-doped multi-cavity Sn/C composite and utilization to anode in lithium ion batteries. Mater. Chem. Phys. 260, 124199 (2020)
Li, N., et al.: Sandwiched N-carbon@Co9S8@Graphene nanosheets as high capacity anode for both half and full lithium-ion batteries. J. Energy Chem. 51, 62–71 (2020)
Goriparti, S., et al.: Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sour. 257, 421–443 (2014)
Mao, C., et al.: Selecting the best graphite for long-life, high-energy Li-ion batteries. J. Electrochem. Soc. 165, A1837 (2018)
Liu, J., Li, G., Wu, J.: Fe2O3–TeO2–MoO3 semiconductor glass-ceramics as anode materials for high specific capacity lithium ion batteries. Mater. Chem. Phys. 258, 123894 (2021)
Li, X., et al.: Effect of dual local structures of amorphous Fe–Si films on the performance of anode of lithium-ion batteries. Mater. Chem. Phys. 243, 122666 (2020)
Luo, J.D., et al.: Agaric-assisted synthesis of core-shell MnO@C microcubes as super-high-volumetric-capacity anode for lithium-ion batteries. Carbon 162, 36–45 (2020)
Ashuri, M., He, Q., Shaw, L.L.: Silicon as a potential anode material for Li-ion batteries: where size, geometry and structure matter. Nanoscale 8, 74–103 (2016)
Li, P., Hwang, J.Y., Sun, Y.K.: Nano/Microstructured Silicon-Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 13, 2624–2633 (2019)
Suh, S., Choi, H., Kim, H.J., Eom, K.: Enhancing the electrochemical properties of a Si anode by introducing cobalt metal as a conductive buffer for lithium-ion batteries. J. Alloys Compd. 827, 154102 (2020)
Park, J., Suh, S., Jeong, S., Kim, H.J.: New approach for the high electrochemical performance of silicon anode in lithium-ion battery: A rapid and large surface treatment using a high-energy pulsed laser. J. Power Sour. 491, 229573 (2021)
Park, S.W., et al.: Enhanced capacity retention based silicon nanosheets electrode by CMC coating for lithium-ion batteries. Electron. Mater. Lett. 17, 268–276 (2021)
Yoo, J.K., et al.: Glyoxalated polyacrylamide as a covalently attachable and rapidly cross-linkable binder for Si electrode in lithium ion batteries. Electron. Mater. Lett. 13, 136–141 (2017)
Gu, M., He, Y., Zheng, J., Wang, C.: Nanoscale silicon as anode for Li-ion batteries: The fundamentals, promises, and challenges. Nano Energy 17, 366–383 (2015)
Szczech, J.R., Jin, S.: Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 4, 56–72 (2011)
Zhang, X., Hayashida, R., Tanaka, M., Watanabe, T.: Synthesis of carbon-coated silicon nanoparticles by induction thermal plasma for lithium ion battery. Powder Technol. 371, 26–36 (2020)
Guo, Y., et al.: Preparation of Rice Husk-Based C/SiO2 Composites and Their Performance as Anode Materials in Lithium Ion Batteries. J. Electron. Mater. 49, 1081–1089 (2020)
Sun, S., et al.: Improved adhesion of cross-linked binder and SiO2-coating enhances structural and cyclic stability of silicon electrodes for lithium-ion batteries. J. Power Sour. 454, 227907 (2020)
Doh, C.H., et al.: A new SiO/C anode composition for lithium-ion battery. J. Power Sour. 179, 367–370 (2008)
Nagao, Y., et al.: Structural analysis of pure and electrochemically lithiated SiO using neutron elastic scattering. J. Electrochem. Soc. 151, A1572 (2004)
Zhang, Z., Huang, Q., Ma, W., Li, H.: Interfacial engineering of polyhedral carbon@hollowed carbon@SiO2 nanobox with tunable structure for enhanced lithium ion battery. Appl. Surf. Sci. 538, 148039 (2021)
Park, D., et al.: Microstructure design of carbon-coated Nb2O5–Si composites as reversible Li storage materials. Electron. Mater. Lett. 16, 376–384 (2020)
Kim, K., et al.: Si-SiOx-Al2O3 nanocomposites as high-capacity anode materials for Li-ion batteries. Electron. Mater. Lett. 13, 152–159 (2017)
Zhang, X., et al.: Facile fabrication of SiO2 nanotubes coated with nitrogen-doped carbon layers as high-performance anodes for lithium-ion batteries. Ceram. Int. 47, 1373–1380 (2021)
Wang, H., et al.: Highly reversible and fast lithium storage in graphene-wrapped SiO2 nanotube network. ChemElectroChem 2, 508–511 (2015)
Xia, T., et al.: Built-in electric field-assisted surface-amorphized nanocrystals for high-rate lithium-ion battery. Nano Lett. 13, 5289–5296 (2013)
Yao, Y., et al.: Carbon-coated SiO2 nanoparticles as anode material for lithium ion batteries. J. Power Sour. 196, 10240–10243 (2011)
Xia, H., Yin, Z., Zheng, F., Zhang, Y.: Facile synthesis of SiO2/C composites as anode materials for lithium-ion batteries. Mater. Lett. 205, 83–86 (2017)
Ali, S., et al.: Photo cured 3D porous silica-carbon (SiO2–C) membrane as anode material for high performance rechargeable Li-ion batteries. J. Alloy. Compd. 812, 152127 (2020)
Aqeel, S.M., et al.: Polyvinylidene fluoride (PVDF)/polyacrylonitrile (PAN)/carbon nanotube nanocomposites for energy storage and conversion. Adv. Comp. hybrid Mater. 1, 185–192 (2018)
Xun, S., Song, X., Battaglia, V., Liu, G.: Conductive polymer binder-enabled cycling of pure tin nanoparticle composite anode electrodes for a lithium-ion battery. J. Electrochem. Soc. 160, A849 (2013)
Eom, J.Y., Cao, L.: Effect of anode binders on low-temperature performance of automotive lithium-ion batteries. J. Power Sour. 441, 227178 (2019)
Cao, S., et al.: In situ carbonized cellulose-based hybrid film as flexible paper anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 8, 1073–1079 (2016)
Li, Z., et al.: A new battery process technology inspired by partially carbonized polymer binders. Nano Energy 67, 104234 (2020)
Piper, D.M., et al.: Conformal coatings of cyclized-PAN for mechanically resilient Si nano-composite anodes. Adv. Energy Mater. 3, 697–702 (2013)
Chen, T., et al.: High performance binder-free SiOx/C composite LIB electrode made of SiOx and lignin. J. Power Sources 362, 236–242 (2017)
Cho, H., Kim, K., Park, C.M., Jeong, G.: Partially carbonized poly (acrylic acid) grafted to carboxymethyl cellulose as an advanced binder for Si anode in Li-ion batteries. J. Electrochem. Sci. Technol. 10, 131–138 (2019)
Shen, X., et al.: Synthesis and anodic performance of TiO2-carbonized PAN electrode for lithium ion batteries. Chem. Phys. 530, 110639 (2020)
Han, Y.J., Park, S.J.: Hydrogen storage behaviors of porous carbons derived from poly (vinylidene fluoride). J. Nanosci. Nanotechnol. 17, 8075–8080 (2017)
Yang, Y., et al.: Highly porous electrospun polyvinylidene fluoride (PVDF)-based carbon fiber. Carbon 49, 3395–3403 (2011)
Habedank, J.B., et al.: Increasing the discharge rate capability of lithium-ion cells with laser-structured graphite anodes: Modeling and simulation. J. Electrochem. Soc. 165, A1563 (2018)
Lee, Y.J., et al.: Fabrication of macroporous Si alloy anodes using polystyrene beads for lithium ion batteries. J. Appl. Electrochem. 46, 695–702 (2016)
Zhang, Y., et al.: Sodium storage in fluorine-rich mesoporous carbon fabricated by low-temperature carbonization of polyvinylidene fluoride with a silica template. RSC Adv. 6, 110850–110857 (2016)
Zhao, D., Sun, J., Li, Q., Stucky, G.D.: Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mat. 12, 275 (2000)
Kruk, M., Jaroniec, M., Ko, C.H., Ryoo, R.: Characterization of the porous structure of SBA-15. Chem. Mat. 12, 1961–1968 (2000)
Li, J.H., et al.: The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 265, 663–670 (2013)
Cao, Y., et al.: Poly(vinylidene fluoride) derived fluorine-doped magnetic carbon nanoadsorbents for enhanced chromium removal. Carbon 115, 503–514 (2017)
Li, Y., et al.: Exploring electrochemistry and interface characteristics of lithium-ion cells with Li1.2Ni0.15Mn0.55Co0.1O2 positive and Li4Ti5O12 negative electrodes. J. Electrochem. Soc. 162, A7049 (2015)
Gong, T., et al.: N, F-codoped microporous carbon nanofibers as efficient metal-free electrocatalysts for ORR. Nano-Micro Lett. 11, 9 (2019)
Lee, J.H., et al.: Property control of graphene by employing “semi-ionic” liquid fluorination. Adv. Funct. Mater 23, 3329–3334 (2013)
Kong, L., Chen, W.: Ionic liquid directed mesoporous carbon nanoflakes as an effiencient electrode material. Sci. Rep. 5, 1–9 (2015)
Lv, P., et al.: Facile preparation and electrochemical properties of amorphous SiO2/C composite as anode material for lithium ion batteries. J. Power Sour. 237, 291–294 (2013)
Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP), the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea [Grant No. 20204010600340] and a GIST Research Institute (GRI) grant funded by the GIST in 2022.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial or other interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original publication, the abstract for this article was inadvertently truncated after the text ‘while the typical SiO2 anode is electrochemically inactive with Li+ ions’. The abstract has been corrected.
Rights and permissions
About this article
Cite this article
Suh, S., Han, S., Yoon, H. et al. Facile One-Step Fabrication of 3-Dimensional SiO2-C Electrodes for Lithium-ion Batteries Using a Highly Porous SBA-15 Template and Pore-Forming Agent. Electron. Mater. Lett. 18, 187–196 (2022). https://doi.org/10.1007/s13391-021-00332-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-021-00332-6