Abstract
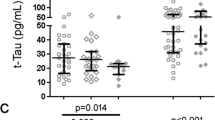
This study aimed to compare serum amyloid processing biomarkers among HIV subtype B (n = 25), HIV subtype C (n = 26), healthy HIV-negative controls (n = 18), and patients with Alzheimer’s disease (AD; n = 24). Immunoassays were used to measure main soluble Aβ isoforms Aβ38, Aβ40, Aβ42, and Aβ-total in serum and cerebrospinal fluid (CSF). People living with HIV (PLWH) and HIV(−) samples, including AD samples, were compared for gender and age, while HIV subtypes were compared for nadir CD4 and plasma viral load suppression. CSF/serum ratios of Aβ40, Aβ42, and Aβ-total were lower in HIV-1C group than in HIV-1B group (p = 0.020, 0.025, and 0.050, respectively). In serum, these biomarkers were comparable. Serum Aβ isoforms were significantly lower in PLWH than in AD. Serum Aβ42 levels in PLWH were decreased compared to those in control group, thus similar to Aβ42 alterations in CSF; these results were different from those observed in AD. Impaired cellular immunity, low CD4 cell count (nadir or current) influences serum Aβ metabolism in HIV-1B but not HIV-1C. However, in PLWH overall, but not in individual HIV subtype groups, greater CD4 recovery, calculated as the difference between current and nadir CD4, correlated with Aβ42/Aβ40 ratio in serum (rs 0.246; p = 0.0479). No significant correlation was found with global deficit score (GDS), an index of neurocognitive performance, age, or duration of infection. These findings are consistent with those of subtype-dependent amyloid processing in blood in chronic HIV disease.



Similar content being viewed by others
References
Aksenov MY, Aksenova MV, Mactutus CF et al (2010) HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett 475:174–178
de Almeida SM, Ribeiro CE, de Pereira AP et al (2013) Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol 19:550–556
de Almeida SM, Tang B, Ribeiro CE et al (2018a) Neprilysin in the cerebrospinal fluid and serum of patients infected with HIV1-subtypes C and B. J Acquir Immune Defic Syndr 78:248–256
de Almeida SM, Ribeiro CE, Rotta I et al (2018b) Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patients infected with HIV-1 subtypes B and C. J Neurovirol 24:28–40
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. DSM-5. Washington, DC: American Psychiatric Association
Andras IE, Toborek M (2013) Amyloid beta accumulation in HIV-1-infected brain: the role of the blood brain barrier. IUBMB Life 65:43–49
Brasil, Ministério da Saúde (2017) Programa Nacional de DST/AIDS. http://www.aids.gov.br/assistencia/manualdst/item12.htm. Accessed 20 Dec 2018
Brew BJ, Pemberton L, Blennow K et al (2005) CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 65:1490–1492
Brown LAM, Jin J, Ferrell D et al (2014) Efavirenz promotes b-secretase expression and increased Ab1–40,42 via oxidative stress and reduced microglial phagocytosis: implications for HIV associated neurocognitive disorders (HAND). PLoS One 9:e95500. https://doi.org/10.1371/journal.pone.0095500
Bu XL, Xiang Y, Jin WS et al (2017) Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol Psychiatry 00:1–9
Chen M, Inestrosa NC, Ross GS et al (1995) Platelets are the primary source of amyloidβ-peptide in human blood. Biochem Biophys Res Commun 213:96–103
Citron M, Vigo-Pelfrey C, Teplow DB et al (1994) Excessive production of amyloid beta-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc Natl Acad Sci U S A 91:11993–11997
Daily A, Nath A, Hersh LB (2006) Tat peptides inhibit neprilysin. J Neurovirol 12:153–160
Deane R, Zlokovic BV (2007) Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 4:191–197
Deane R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of amyloid-β peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8:16–30
El Ali A, Rivest S (2013) The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease. Front Physiol 4:45. https://doi.org/10.3389/fphys.2013.00045
Esiri MM, Biddolph SC, Morris CS (1998) Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry 65:29–33
Evin G, Zhu A, Holsinger RM et al (2003) Proteolytic processing of the Alzheimer’s disease amyloid precursor protein in brain and platelets. J Neurosci Res 74:386–392
Gisslén M, Krut J, Andreasson U et al (2009) Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol 9:63
Giunta B, Zhou Y, Hou H et al (2008) HIV-1 TAT inhibits microglial phagocytosis of Abeta peptide. Int J Clin Exp Pathol 1:260–275
Graff-Radford NR, Crook JE, Lucas J et al (2007) Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol 64:354–362
Green DA, Masliah E, Vinters HV et al (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19:407–411
Gross AM, Jaeger PA, Kreisberg JF et al (2016) Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 62:157–168
Hamel F (2007) HIV Protease inhibitors inhibit insulin-degrading enzyme (IDE) function. 67th Scientific Sessions. American Diabetes Association, Chicago, (poster 2624)
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
High KP, Brennan-Ing M, Clifford DB et al (2012) HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 60:S1–S18
Johnston JA, Liu WW, Coulson DT et al (2008) Platelet beta-secretase activity is increased in Alzheimer’s disease. Neurobiol Aging 29:661–668
van der Kant R, Goldstein LSB (2015) Cellular functions of the amyloid precursor protein from development to dementia. Dev Cell 32:23
Kim J, Yoon JH, Kim YS (2013) HIV-1 Tat interacts with and regulates the localization and processing of amyloid precursor protein. PLoS One 29(8):e77972
Knight R, Khondoker M, Magill N, Stewart R, Landau S (2018) A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement Geriatr Cogn Disord 45:131–151
Krut JJ, Zetterberg H, Blennow K et al (2013) Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol 260:620–626
Krut JJ, Gisslen M, Hagberg L, et al (2014) Hyperphosphorylated Tau in cerebrospinal fluid: a biomarker for neurological aging in HIV? Program and abstracts of the 21th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 3-6 March (poster 453)
Kuo YM, Kokjohn TA, Watson MD et al (2000) Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol 156:797–805
Lan X, Xu J, Kiyota T et al (2011) HIV-1 reduces Abeta-degrading enzymatic activities in primary human mononuclear phagocytes. J Immunol 186:6925–6932
Lan X, Kiyota T, Hanamsagar R et al (2012) The effect of HIV protease inhibitors on amyloid-β peptide degradation and synthesis in human cells and Alzheimer’s disease animal model. J NeuroImmune Pharmacol 7:412–423
Letendre S, Ellis R, Deutsch R, et al (2010) Correlates of time-to-loss-of-viral response in CSF and plasma in the CHARTER cohort. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 16–19 February (poster 430)
Lewczuk P, Matzenc A, Blennowd K et al (2017) Cerebrospinal fluid AB42/40 corresponds better than AB42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis 55:813–822
Liu WW, Todd S, Craig D et al (2007) Elevated platelet beta-secretase activity in mild cognitive impairment. Dement Geriatr Cogn Disord 24:464–468
Lopez OL, Kuller LH, Mehta PD et al (2008) Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 70:1664–1671
Mankowski JL, Queen SE, Tarwater PM et al (2002) Accumulation of b-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol 61:85–90
Marks KM, Clarke RM, Bussel JB et al (2009) Risk factors for thrombocytopenia in HIV-infected persons in the era of potent antiretroviral therapy. J Acquir Immune Defic Syndr 52:595–599
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Mehta PD, Pirttila T, Mehta SP et al (2000) Plasma and cerebrospinal fluid levels of amyloid BProteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 57:100–105
Molinuevo JL, Gispert JD, Dubois B et al (2013) The AD-CSF-Index discriminates Alzheimer’s disease patients from healthy controls: a validation study. J Alzheimers Dis 36:67–77
Mooney S, Tracy R, Osler T et al (2015) Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher Framingham and VACS risk index scores. PLoS One 10:e0144312
Nath A, Hersh LB (2005) Tat and amyloid: multiple interactions. AIDS 19:203–204
Noel N, Boufassa F, Lecuroux C et al (2014) Elevated IP10 levels are associated with immune activation and low CD4(+) T-cell counts in HIV controller patients. AIDS 28:467–476
van Oijen M, Hofman A, Soares HD et al (2006) Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 5:655–660
Peterson J, Gisslen M, Zetterberg H et al (2014) Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 9:e116081. https://doi.org/10.1371/journal.pone.0116081
Pulliam L (2009) HIV regulation of amyloid beta production. J NeuroImmune Pharmacol 4:213–217
Raboni SM, Ribeiro CE, Almeida SM et al (2017) Impact of public health strategies on reducing AIDS mortality in southern Brazil. Int J STD AIDS 28:54–62
Rempel HC, Pulliam L (2005) HIV-1Tat inhibits neprilysin and elevates amyloid beta. AIDS 19:127–135
Toledo JB, Shaw LM, Trojanowski JQ (2013) Plasma amyloid beta measurements—a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res Ther 5:8. https://doi.org/10.1186/alzrt162
Tran H, Robinson S, Mikhailenko I et al (2003) Modulation of the LDL receptor and LRP levels by HIV protease inhibitors. J Lipid Res 44:1859–1869
Van Nostrand WE, Melchor JP (2001) Disruption of pathologic amyloid beta-protein fibril assembly on the surface of cultured human cerebrovascular smooth muscle cells. Amyloid 8:20–27
Wang MJ, Yi SH, Han J et al (2017) Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimers Res Ther 9:98
White JA, Manelli AM, Holmberg KH et al (2005) Differential effects of oligomeric and fibrillar amyloid-beta 1–42 on astrocyte-mediated inflammation. Neurobiol Dis 18:459–465
Wood SN (2006) Generalized additive models: an introduction with R. CRC Press, LLC, Boca Raton
Xu J, Ikezu T (2009) The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J NeuroImmune Pharmacol 4:200–212
Zhang J, Liu J, Katafiasz B et al (2011) HIV-1 gp120- induced axonal injury detected by accumulation of beta-amyloid precursor protein in adult rat corpus callosum. J NeuroImmune Pharmacol 6:650–657
Acknowledgments
This work was supported by the following grants: National Institute of Health, NIH R21 MH76651 (Ellis, Ronald J; Almeida, Sergio M.), S10 RR31646 (Letendre, Scott), K24 MH097673(Letendre, Scott); University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Bin Tang, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Study funding
This study was funded by CFAR (International Pilot Grant P30 AI036214 and CFAR Visiting Researcher Grant PTHMON7) and NIMH (R21 MH076651-01).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Almeida, S.M., Ribeiro, C.E., Rotta, I. et al. Blood amyloid-β protein isoforms are affected by HIV-1 in a subtype-dependent pattern. J. Neurovirol. 26, 3–13 (2020). https://doi.org/10.1007/s13365-019-00783-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-019-00783-6