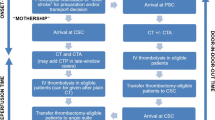
Abstract
Stroke is an urgent public health issue with millions of patients worldwide living with its devastating effects. The advent of thrombolysis and endovascular thrombectomy has transformed the hyperacute care of these patients. However, a significant proportion of patients receiving these therapies still goes on to have unfavorable outcomes and many more remain ineligible for these therapies based on our current guidelines. The future of stroke care will depend on an expansion of the scope of thrombolysis and endovascular thrombectomy to patients outside traditional time windows, more distal occlusions, and large vessel occlusions with mild clinical deficits, for whom clinical trial results have not proven therapeutic efficacy. Novel cytoprotective therapies targeting the ischemic cascade and reperfusion injury therapy, in combination with our existing treatment modalities, should be explored to further improve outcomes for these patients with acute ischemic stroke. In this review, we will review the current status of thrombolysis and thrombectomy, suggest additional data that is needed to enhance these therapies, and discuss how cytoprotection might be combined with thrombectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke remains a substantial public health issue. Worldwide, it ranks as the second leading cause of death in adults, and it is a leading cause of long-term physical and cognitive disability. Over 12 million new strokes occur each year, and more than 100 million people are living with the effects of stroke. Economically, the impact is in the trillions of dollars annually [1]. Large vessel occlusions (LVOs) account for up to 38% of these strokes [2]. Though the hyperacute care of patients with ischemic stroke has been transformed by the advent of thrombolysis and endovascular thrombectomy (EVT), many patients continue to have unfavorable outcomes despite receiving these therapies and many more remain ineligible for these treatments based on current guidelines. A study analyzing data from the American Heart Association’s Get with the Guidelines-Stroke registry found that the rate of EVT for LVOs increased from 2.2% in 2015 to 24.2% in 2018 [3]. However, despite successful recanalization, only approximately 50% of patients undergoing EVT have a favorable outcome [4]. Herein, we discuss the current landscape of acute ischemic stroke therapy and a framework for future directions with a focus on potentially combining cytoprotection with EVT to improve outcomes.
Overview of the Therapeutic Efficacy of Mechanical Thrombectomy and Lack Thereof
Anterior Circulation Ischemic Strokes
The initial landmark trials demonstrating the clinical benefit of EVT in acute ischemic stroke (AIS) secondary to large vessel occlusions (LVOs) were MR CLEAN [5] (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands), ESCAPE [6] (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times), EXTEND IA [7] (Extending the Time for Thrombolysis in Emergency Neurological Deficits — Intra-Arterial), SWIFT PRIME [8] (Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment), REVASCAT [9] (Randomized Trial of Revascularization with Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting within Eight Hours of Symptom Onset), and THRACE [10] (Mechanical Thrombectomy After Intravenous Alteplase Versus Alteplase Alone After Stroke). During the last decade, the management of AIS with EVT has shown remarkable outcomes due to the utilization of EVT along with intravenous (IV) tissue-plasminogen activator (tPA). In summary, these six clinical trials enrolled 1701 patients in aggregate and provided level 1A evidence in favor of EVT in AIS patients with LVO treated at comprehensive stroke centers (CSC). The numbers needed to treat (NNT) of 3–7 demonstrated the effectiveness of EVT in providing an independent level of neurological functioning. The DAWN [11] (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) and DEFUSE-3 [12] (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trials included patients with an extended time window (6–24 h) from stroke-onset for EVT and provided further evidence for the efficacy and safety of EVT in AIS due to LVOs in the anterior circulation in carefully selected patients. The DEFUSE-3 and DAWN trials provide level IA and level IIB evidence, respectively, and this is reflected in current stroke guidelines recommending EVT in the late window with adherence to the protocols of these two trials. A recently published systematic review and individual patient data meta-analysis further strengthens the evidence for the benefit of EVT in patients presenting within 6–24 h of stroke onset with evidence of reversible cerebral ischemia [13].
Posterior Circulation Ischemic Strokes
The data on posterior circulation ischemic stroke is scarce, and there is very limited utility of the Alberta Stroke Program Early CT Score (ASPECTS), computed tomography (CT) perfusion (CTP), and magnetic resonance imaging (MRI) in these strokes [14]. The BASICS [15] (Basilar Artery International Cooperation Study) registry could not exclude a benefit for EVT in basilar artery occlusion (BAO) related stroke presentations. An earlier trial, BEST (Endovascular Treatment vs. Standard Medical Treatment for Vertebrobasilar Artery Occlusion), failed to show any favorable outcomes in EVT for BAO. However, this trial was limited by a small sample size, failure to adhere to the study protocol, and early termination [16]. More recently, the BAOCHE [17] (Basilar Artery Occlusion Chinese Endovascular) trial in a Chinese Han population showed an encouraging outcome for EVT in AIS due to BAO in the extended-time window (6–24 h). Mortality at 90 days was 31% in the EVT group and 42% in the control group, with 6% of patients experiencing bleeding complications in the EVT group compared to 1% in the control group. The trial showed a better functional outcome at 90 days, but increased hemorrhage risk in the EVT group. Similar results were also shown in the ATTENTION [18] (Endovascular Treatment for Acute Basilar Artery Occlusion — a Multicenter Randomized Clinical Trial) trial where EVT was done within 12 h of BAO-related strokes in 36 centers across China. These trials were terminated prematurely when the evidence suggested convincing treatment efficacy of EVT in posterior circulation AIS. The key features of the trials discussed above are summarized in Tables 1 and 2 for anterior and posterior circulation strokes, respectively. It is not known if EVT has a similar safety and efficacy profile in patients on anticoagulants. A small study showed better outcomes with EVT in patients on direct oral anticoagulants (DOACs) as compared to patients taking vitamin K antagonists (VKAs) [19].
Future Perspectives
In summary, EVT is a safe procedure in carefully selected patients with a modified Rankin Scale (mRS) score < 3 before their stroke. However, in this current era of endovascular treatment of AIS, there is still some hesitancy among interventionalists in offering EVT to patients with severe clinical deficits and low ASPECTS or a large core on CTP imaging. This was primarily due to a lack of supporting data in these patients. An initial retrospective cohort study of patients with LVO who underwent EVT with a low ASPECTS score of 2–5 showed good functional outcomes in 1 of 5 patients [20]. More recently, the SELECT-2 [21] (Trial of Endovascular Thrombectomy for Large Ischemic Strokes) and ANGEL-ASPECT [22] (Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct) trials demonstrated better functional outcomes with EVT compared to standard medical care in patients with a low ASPECTS score of 3–5 presenting within 24 h of stroke onset. Both of these trials randomized patients with baseline mRS 0–1and a large core as determined by ASPECTS 3–5 (a few cases of ASPECTS 0–2 or > 5 were include in ANGEL-ASPECT based on perfusion data) or CTP core > 50 mL (SELECT-2)/70–100 mL (ANGEL-ASPECT). There are currently other ongoing “large core” trials that we are aware of. However, the publication of these recent large core trials is expected to shift the paradigm toward offering EVT to patients with larger ischemic cores.
Conversely, there is a lack of data on whether EVT is beneficial in patients presenting with an LVO and a low National Institutes of Health Stroke Scale (NIHSS) score. A pooled analysis of 3 prospective international cohorts included patients with an LVO presenting within 12 h of stroke onset and NIHSS \(\le 6.\) Though EVT and medical management resulted in similar proportions of excellent clinical outcomes at 90 days, EVT was associated with an increased risk of neurologic deterioration at 24 h [23]. Similar early neurologic deterioration, but without significant clinical benefit, was reported in two other studies enrolling patients with an LVO and NIHSS < 5 [24, 25]. Currently, the ENDOLOW [26] (Endovascular Therapy for Low NIHSS Ischemic Strokes) trial is studying EVT in patients arriving within eight hours of stroke onset with a proximal LVO and a low NIHSS of 0–5.
In the future, the development of more advanced stent systems, especially for the posterior circulation and petrosal internal carotid artery, may decrease bleeding complications and other procedural risks [27]. As more CSCs are being established, nationwide large-datasets and registries can provide more comprehensive and informative data necessary to study the safety and efficacy and long-term outcomes of EVT. Other populations of interest that are currently being explored and for whom 1-day EVT may be considered standard of care include late presentations beyond 24 h and those with medium vessel occlusions (MeVOs) and distal vessel occlusions (DiVOs).
Thrombolysis Before and After Endovascular Thrombectomy
Intravenous Tissue-Plasminogen Activator
Since 1995, IV tPA has been the standard of care for patients arriving within 4.5 h of stroke symptom onset [28, 29]. All patients who are eligible for IV tPA should receive it regardless of whether an indication for thrombectomy exists, as per the 2019 American Heart Association (AHA)/American Stroke Association (ASA) guidelines [30]. Furthermore, the Society of Vascular and Interventional Neurology (SVIN) committee recommends against skipping thrombolysis in otherwise eligible patients presenting with an anterior circulation LVO amenable to EVT [31]. Hence, thrombolysis with IV tPA remains the standard of care. The SKIP [32] trial in Japan failed to show non-inferiority of EVT alone as compared to combining IV tPA and EVT on functional outcomes in patients with AIS due to LVO. Several other clinical trials have also supported this notion that EVT alone is not non-inferior to the combination of EVT and thrombolysis [33,34,35,36]. In patients with minor clinical deficits and a low NIHSS score, IV tPA alone was similarly effective to the combination of IV tPA plus EVT, but the need remains for a larger randomized clinical trial in these patients to replicate this finding [25].
Intra-Arterial Tissue-Plasminogen Activator
In large clinical trials and metanalyses, endovascular interventions including EVT with or without intra-arterial tissue-plasminogen activator (IA tPA) in patients presenting with stroke secondary to LVOs demonstrated benefits in survival and functional neurologic outcomes with a slight risk of intracerebral hemorrhage (ICH), reperfusion injury, or other complications [37]. There is an ongoing debate for using adjunct thrombolysis with IA-tPA after EVT to improve functional outcomes in patients with reperfusion measured by thrombolysis in cerebral infarct (TICI) score of 2b or higher. The application of IA-tPA is limited due to the concern of increased risk of ICH and other complications after reperfusion. A recent phase-2 trial in Spain, CHOICE [38] (CHemical OptImization of Cerebral Embolectomy in Patients With Acute Stroke Treated With Mechanical Thrombectomy), found that IA tPA in adjunct to EVT for LVOs in the anterior, middle, or posterior cerebral arteries improved functional outcomes at 90 days without significantly increasing the risk of ICH. However, these results should be replicated in a larger trial with a more heterogenous population of patients before being adopted into clinical practice.
Tenecteplase
Tenecteplase (TNK) is a newer thrombolytic that has already been adapted for use in thrombolysis of myocardial infarction. More recently, there have been several studies providing robust evidence to support the use of TNK (0.25 mg/kg dose, max dose of 25 mg) in acute ischemic stroke as an alternative to alteplase [39, 40]. TNK is a genetically modified form of tPA that has a longer half-life and less systemic coagulopathy effects owing to its increased specificity for fibrin as opposed to fibrinogen. These factors lead to better recanalization rates and fewer bleeding complications as compared to tPA [41]. For example, the EXTEND-IA TNK [42] (Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke) trial demonstrated better functional outcomes and increased recanalization rate of LVOs with TNK compared to tPA when administered within 4.5 h of stroke onset in patients also undergoing EVT. The safety outcome of symptomatic ICH (~ 1%) was also similar between the two groups. The role of TNK as an intra-arterial adjunct after EVT remains unclear. Overall, TNK is a reasonable alternative to tPA for stroke thrombolysis with potentially better early reperfusion and other practical advantages such as rapidity of drug delivery and lower cost compared to tPA [43]. More recently, the TRACE-2 [44] (Tenecteplase vs Alteplase in Acute Ischaemic Cerebrovascular Events) trial demonstrated non-inferiority of TNK compared to tPA in patients who were ineligible for EVT and showed reassuring functional outcomes with similar symptomatic hemorrhage rates and mortality.
Future Perspectives
In the future, TNK is very likely to become the preferred thrombolytic agent in the standard 4.5-h window with all the available evidence and its inherent advantages compared to tPA. Beyond the standard time window, the TIMELESS [45] (Tenecteplase in Stroke Patients Between 4.5 and 24 Hours) trial is evaluating the safety and efficacy of TNK in an extended window of 4.5–24 h with the aid of advanced imaging to detect the ischemic penumbra. As another potential indication for TNK in stroke, the TEMPO-2 [46] (Trial of TNK-tPA Versus Standard of Care for Minor Ischemic Stroke With Proven Occlusion) trial is studying the role of TNK in minor stroke with distal occlusion up to 12 h after symptom onset after reassuring safety and efficacy data in the smaller TEMPO-1 trial [47]. In an attempt to further optimize the efficacy of thrombolysis in AIS, the MOST [48] (Multi-arm Optimization of Stroke Thrombolysis) trial is randomizing patients receiving standard-of-care thrombolysis (IV tPA or TNK) to also receiving an infusion of argatroban, eptifibatide, or placebo to evaluate if the addition of these agents will improve functional outcomes as traditional thrombolysis is only partially (~ < 30%) effective in achieving and maintaining recanalization. Standard-of-care EVT will also be allowed in patients being enrolled in this trial.
Cytoprotection Overview
During an ischemic insult, the penumbra is an area without electrical activity and impaired protein synthesis, but with preserved ion homeostasis, membrane potentials, and high-energy metabolism [49]. Modern reperfusion strategies, such as thrombolysis and endovascular thrombectomy (EVT), work by affecting the ischemic cascade via the restoration of blood flow supplying oxygen and glucose, and thereby salvaging part of the ischemic penumbra. However, despite successful recanalization, only approximately 50% of patients undergoing EVT have a favorable outcome. This is primarily because a large portion of the ischemic brain tissue has already become irreversibly injured at the time of reperfusion [50]. It is this problem that gives rise to the concept of “protecting” or “freezing” the ischemic penumbra, the area of hypoperfused brain tissue that is at risk of becoming infarcted [51].
One potential strategy in protecting the ischemic penumbra is to directly target components of the ischemic cascade, termed cytoprotection. However, research spanning several decades into a number of different cytoprotective therapies has yet to yield a therapy with a clear benefit despite promising results in animal models. There are several potential explanations for why these therapies have failed to show a benefit in clinical trials (Table 3). One of the most important explanations is the sheer complexity of the ischemic cascade, a series of intracellular events triggered after focal brain ischemia that leads to excitotoxicity, post-ischemic inflammation, oxidative stress, microvascular injury, blood–brain barrier dysfunction, and eventual cell death [52,53,54]. Targeting only one component of this multifaceted pathophysiological process, as cytoprotection trials have done in the past, is unlikely to yield a meaningful clinical benefit [55].
Targets for Cytoprotection
Historically, targeting a single component of the ischemic cascade has been an unsuccessful approach. Future therapies should focus on a multifaceted approach targeting multiple aspects of the ischemic cascade. This is likely best accomplished by targeting the most upstream mechanisms of cellular injury such as glutamate-mediated excitotoxicity, as is accomplished by nerinetide, a recent promising addition to the long list of potential cytotherapeutic agents [56, 57].
The advent of thrombolysis and EVT has revolutionized the hyperacute care of patients of ischemic stroke with more patients achieving recanalization. Though this recanalization leads to good clinical outcomes in some patients, it also gives rise to another potential consequence, reperfusion injury [58, 59]. This complex pathophysiological process is mediated by a number of inflammatory cytokines, matrix metalloproteinases, and endothelial function mediators and ultimately leads to disruption of the blood–brain barrier [60, 61]. Hemorrhagic transformation is the radiologic manifestation of this process in some patients [62, 63]. Preclinical models have demonstrated that reperfusion injury can contribute to cortical damage [64]. This gives rise to yet another important target for the future development and testing of cytotherapeutic drugs.
Though not strictly a target for cytoprotection and more-so a target for thrombolysis, the “no-reflow” phenomenon is relevant in the era of EVT. First described in animal model in the 1960s and 1970s, it is the absence of tissue perfusion despite complete recanalization of an occluded proximal vessel and is thought to be secondary to ultrastructural microvascular damage and resulting microvascular perfusion [65, 66]. This process is thought to be mediated by obstruction of the microvasculature via focal endothelial swelling, neutrophil plugs, platelets, fibrin, and erythrocyte stacking [67, 68]. The role of the collateral circulation in ischemic stroke has recently gained more recognition and there has been a shift from “Time is Brain” to “Imaging is Brain” [69]. Whether the infarct core is relatively small or of modest size can be approximated on a standard head CT through the ASPECTS score [70]. This approach was used in two recently reported studies that demonstrated beneficial effects of EVT up to 24 h after stroke onset with ASPECTS of 3–5 [21, 22]. In contrast, the initial studies demonstrating the benefits of late time-window EVT, DAWN, and DEFUSE-3 used more advanced imaging with CT perfusion or diffusion/perfusion MRI [11, 12]. During focal cerebral ischemia, an extensive collateral circulation system via leptomeningeal collaterals and external carotid to internal carotid collaterals attempts to maintain perfusion, and poor collaterals are associated with worse outcomes and larger infarct volumes [69, 71]. However, collateral vessels are often small and may not be able to maintain blood flow indefinitely. Studies have examined the evolution of collateral vessels over time in patients with stroke and have shown that collateral flow can deteriorate overtime in patients with acute stroke and is associated with infarct growth [72]. This creates yet another target for future cytoprotective therapies with a focus on keeping the collateral circulation open long enough, presuming there are adequate collaterals, for reperfusion strategies to take effect.
Beyond excitotoxicity, failure of the collateral circulation, and reperfusion injury, there is abundant evidence that cerebral ischemia leads to a significant inflammatory response mediated by peripheral leukocytes, endogenous microglia, and a number of inflammatory cytokines [73]. This process is also implicated in the no-reflow phenomenon as animal models have shown neutrophils adhering to distal capillary segments as one factor in microvascular occlusion, and neutrophil depletion via antibodies has been shown to restore microvascular perfusion in mice without increasing the rate of hemorrhagic complications [74]. Furthermore, preclinical stroke models have shown that Fingolimod, a disease-modifying drug approved in relapsing–remitting multiple sclerosis, increases reperfusion by attenuating microvascular thrombus formation [75, 76]. In a small clinical trial evaluating tPA with or without fingolimod in a 4.5- to 6-h time window, fingolimod was found to enhance the efficacy of tPA by promoting anterograde reperfusion and retrograde collateral flow as assessed by 4-D CT angiography [77].
Therapeutic hypothermia (TH), a non-pharmacologic strategy with the potential to target multiple deleterious pathways in ischemia (i.e., neuroinflammation, free radical production, disruption of the blood–brain barrier) [78], has been show in animal studies to reduce infarct size by up to 44% [79]. However, a systematic review and meta-analysis of studies implementing TH in acute ischemic stroke did not find an overall beneficial effect and had increased complications, though there were individual studies that showed a shift toward better outcome [80]. One explanation for this is the time-sensitive nature of implementing TH seen in animal models, where even a delay of 30 min resulted in inadequate neuroprotection [81]. Future trials exploring TH in acute ischemic stroke should aim to implement TH and achieve target temperature earlier in the time window of ischemia.
New Era of Cytoprotection
Almost all of the cytoprotection studies in ischemic stroke were completed in the era before modern reperfusion therapy. Therefore, there is minimal to no data on the effect of these drugs when substantial recanalization has been achieved. The future of cytoprotection in ischemic stroke should rely on combining reperfusion and cytoprotective therapies with the goals of protecting ischemic tissue before reperfusion, protecting injured cells during and after recanalization, ameliorating reperfusion injury, keeping the collateral circulation open, and preventing microvascular injury and occlusion. This can be through the development of novel therapeutics or the repurposing of previously failed cytoprotective drugs.
The phase III ESCAPE-NA1 (Efficacy and Safety of Nerinetide for the Treatment of Acute Ischemic Stroke) trial of nerinetide in patients with ischemic stroke undergoing EVT provided promising results and renewed hope that cytoprotection may have meaningful clinical benefit [82]. Nerinetide, a postsynaptic density-95 (PSD-95) inhibitor, works by inhibiting the signaling interaction of NMDA receptors with the PSD-95 submembrane scaffolding protein. This is believed to inhibit neurotoxic NMDA-receptor signaling and free radical production without inhibiting necessary glutamatergic neurotransmission [83]. Several animal models involving rats and primates demonstrated significant clinical benefit and provided a strong rationale to move forward with clinical trials in humans [84]. Prior to the ESCAPE-NA1 trial, the ENACT (Safety and Efficacy of NA-1 in Patients with Iatrogenic Stroke After Endovascular Aneurysm Repair) phase II trial studied nerinetide in patients undergoing endovascular treatment of ruptured or unruptured intracranial aneurysm. Patients who received nerinetide had fewer ischemic infarcts as a complication of the procedure, thus demonstrating that neuroprotection in humans was possible, although it must be acknowledged that most of the infarcts seen on MRI were very small [85].
Cytoprotection as an Adjunct to EVT
The ESCAPE-NA1 trial heralded a new era of cytoprotection as it combined it with EVT. In this trial, patients with moderate-to-good collaterals and a favorable CT scan undergoing EVT were randomized to receive a single intravenous dose of nerinetide or placebo prior to EVT. A total of 59.2% of patients in the placebo arm and 60.1% of patients in the nerinetide arm also received intravenous alteplase. The primary efficacy outcome, a score of 0–2 on the modified Rankin scale, did not differ between the two arms (59.2% in the placebo arm vs. 61.4% in the nerinetide arm; adjusted risk ratio 1.04, 95% CI 0.96–1.14; p = 0.35). However, there was a statistically significant difference in favorable outcome based on a subgroup analysis, defined a priori, as those patients who did not receive tPA prior to randomization (adjusted risk ratio 1.18, 95% CI 1.01–1.38). This difference in outcomes between those who received and did not receive tPA appears to be explained, at least part, by the fact that peak serum levels of nerinetide in patients who also received tPA were significantly lower than those who did not receive tPA. It was later revealed, through animal models, that plasmin whose levels are increased by tPA administration cleaves nerinetide [86]. Other potential explanations for the negative primary outcome were the facts that advanced perfusion imaging was not performed to assess the volume of penumbra and the nerinetide was only administered just before the patient was taken for EVT. Given the mechanism of action of nerinetide and what we understand about the ischemic penumbra, it is safe to presume that the earlier in the stroke the medication is delivered, the better the potential outcome. Nonetheless, the results of this trial and its subgroup analysis are promising for the future of nerinetide and cytoprotection. A phase III trial of nerinetide in patients undergoing EVT who have not received tPA is currently underway (ESCAPE-NEXT; NCT04462536) and should report results soon.
In addition to nerinetide, other cytoprotective drugs have been evaluated in conjunction with reperfusion therapy. The recently reported phase Ib/IIa trial of ApTOLL, a toll-like receptor antagonist, enrolled patients undergoing EVT within 6 h of stroke onset with an ASPECTS of 5–10 and ischemic core volume of 5–70 mL on advanced imaging [87]. The higher dose of the study drug improved the 90-day outcome on the mRS shift analysis and also reduced 90-day mortality. Nelonemdaz, a cytoprotective drug with multiple mechanisms of action including reducing calcium permeability of the N-methyl-D-aspartate receptor and scavenging of oxygen free radicals, was evaluated in a phase-2 clinical trial [88]. Two doses of nelonemdaz were compared to placebo in patients undergoing EVT within 8 h of stroke onset. No differences were seen in the primary outcome, mRS 0–2, among the groups. A nonpharmacological cytoprotective treatment, normobaric hyperoxia, was studied in patients undergoing EVT [89]. Giving high-flow oxygen supplementation with EVT was associated with a reduction of follow-up infarct size on MRI and an improved 90-day mRS outcome in this relatively small trial. Uric acid, a potent antioxidant, was evaluated in a moderately sized clinical trial in which all patients also received tPA and a small percentage (11%) also underwent thrombectomy [90]. The trial did not demonstrate an overall significant difference between the treated and placebo groups, but interestingly, the female participants who had lower baseline uric acid levels did have a significantly better outcome with uric acid, as did patients with elevated glucose levels at stroke onset. Interestingly, a recent report from the Stroke Preclinical Assessment Network (SPAN) identified uric acid as the most promising cytoprotective approach in a well-designed series of preclinical experiments at multiple testing sites [91]. These results and the prior clinical trial suggest that uric acid is a promising candidate for future late-stage clinical trials with EVT, especially in women. In addition to these agents, a number of other ones are being evaluated in early phase clinical trials with EVT as outlined in a recent excellent review of topic [92].
Design of Future Trials
A number of different approaches can be envisioned for combining cytoprotection with EVT as outlined in Table 4. The nerinetide clinical trials provide guidance for a trial that administers cytoprotection just before EVT at a facility that can perform endovascular treatment. If the current nerinetide clinical trial in patients who have not received tPA before thrombectomy, ESCAPE-NEXT, is positive, it is likely that it will be the first cytoprotective drug to be approved for use in clinical practice. As discussed before, patients in the nerinetide clinical trials received the study drug a short time before undergoing thrombectomy, so it is unlikely that nerinetide had much effect on ischemic brain tissue before large artery recanalization occurred. Future clinical trials with other study drugs can be envisioned in which randomization and drug initiation occur just prior to recanalization or shortly thereafter. These trials will be challenging because patient selection and informed consent will take place as the patient is being prepared for thrombectomy which is being performed increasingly quickly after patients arrive at a thrombectomy capable center.
Two types of clinical trials can be envisioned before patients arrive at an EVT capable center [93]. One such trial would enroll patients at a smaller, outlying hospital who is identified as a likely thrombectomy candidate by the demonstration of a LVO on CT angiography and the exclusion of a large infarct core on a head CT scan. The addition of a CTP study could potentially enhance patient selection for such a trial because CTP can provide an approximation of the extent of ischemic penumbra and also identify patients in whom the ischemic core may enlarge more rapidly (i.e., “fast progressors”) [94]. Unfortunately, CTP is not widely available at many smaller hospitals, but there are an increasing number of hub and spoke hospital networks where CTP is available at spoke hospitals. With the availability of telemedicine consultations and commercial imaging analysis software, the stroke patients in the emergency departments of smaller hospitals can be evaluated by stroke specialists and the acquired CT studies can be accurately evaluated [95]. These capabilities will enhance enrollment and patient selection for clinical trials that enroll patients at outlying hospitals before transfer to a thrombectomy capable center. The primary goal of such a trial would be to slow down the progression of the ischemic penumbra into the ischemic core during transport. Patients will need to be re-imaged at the EVT center before undergoing thrombectomy with CTP or MRI to determine the effects of the cytoprotective drug on ischemic core growth. Demonstrating a reduction of ischemic core growth would provide strong supportive evidence that the therapy being studied actually was protecting the brain from ischemic injury during transport. A secondary goal would be to determine the percentage of patients who remain good EVT candidates. Because the primary outcome of a hub and spoke trial is based on imaging, it will be most appropriate as a phase II trial, and a similar imaging endpoint could be used in a phase III trial as a secondary outcome measure. This type of trial will, however, provide proof-of-concept data that the study drug is cytoprotective.
A second type of trial evaluating patients during transport to a thrombectomy center would be in the ambulance. In such a trial, patients likely to have an LVO can be identified based upon the severity of the stroke using the NIHSS where a score of 10 or greater strongly suggests the presence of a proximal vessel occlusion [96]. Telemedicine consultation with a stroke specialist would enhance the accuracy of the clinical evaluation. Additionally, technologies such as electroencephalography and somatosensory evoked potentials are being developed for use in ambulances that could provide additional useful information for LVO identification [97]. Mobile stroke units with onboard imaging capability, typically CT, are now available in a limited number of locations [98]. These units would be ideal for ambulance based clinical trials of cytoprotective drugs before thrombectomy. The best primary outcome measure for such ambulance-based trials is uncertain, but one possibility would be to assess penumbral and infarct core size upon arrival at the thrombectomy center.
Another approach to clinical trials of cytoprotection with thrombectomy would be to study molecules that target reperfusion injury and to randomize patients after thrombectomy induced substantial or complete LVO recanalization [4]. Until recently, reperfusion injury did not have much clinical relevance because tPA has only modest recanalization/reperfusion efficacy, so secondary tissue injury was likely modest, but now with the advent of effective thrombectomy induced vessel opening, it has increasing potential importance. In trials targeting reperfusion injury, the study drug should be one that has a mechanism of action relevant to this type of injury. In a reperfusion injury targeted clinical trial, only patients with confirmed substantial or complete recanalization should be included (i.e., the TICI score should be 2b, 2c, or 3). Advanced imaging with CT or MRI should also be used to evaluate the extent of the ischemic core and penumbra because patients with large ischemic cores or little to no ischemic penumbra are unlikely to benefit from cytoprotection started after successful reperfusion. Patients with hemorrhagic transformation after thrombectomy should also be excluded with either CT or MRI. Such reperfusion injury targeted trials will be difficult to perform and will likely require a large sample size because all the patients randomized will have undergone a successful thrombectomy, and the treatment effect in the control group receiving placebo will be substantial. The primary outcome will be clinical efficacy such as the 90-day mRS. A signal of efficacy in earlier phase trials might be detected on NIHSS at day 7 [99] or the mRS at day 30. Imaging markers of efficacy such as infarct growth over several days could also be assessed.
Conclusion
Since the advent of thrombolysis in 1995, significant leaps have been made in our ability to treat acute ischemic stroke, especially with EVT becoming standard of care in 2015. Despite these advances, many stroke patients still go on to have unfavorable outcomes. However, the many ongoing trials of endovascular treatments to expand the pool of eligible patients and the potential use of adjunctive cytoprotective therapies provide hope for additional tools in our fight to improve stroke outcomes.
References
Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29.
Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40(12):3834–40.
Adeoye O, Albright KC, Carr BG, Wolff C, Mullen MT, Abruzzo T, et al. Geographic access to acute stroke care in the United States. Stroke. 2014;45(10):3019–24.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138–47.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–18.
Jovin TG, Nogueira RG, Lansberg MG, Demchuk AM, Martins SO, Mocco J, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet. 2022;399(10321):249–58.
Ikram A, Farooqui M, Suriya S, Quadri SA, Zafar A. Smog sign: hazy diffusion-weighted imaging restriction in dense axonal tracts in the pons on hyperacute MRI with remarkable clinical improvement after intra-arterial thrombectomy. Cureus. 2019;11(8):e5461.
Langezaal LCM, van der Hoeven E, Mont’Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384(20):1910–20.
Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115–22.
Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387(15):1373–84.
Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387(15):1361–72.
Chen JH, Hong CT, Chung CC, Kuan YC, Chan L. Safety and efficacy of endovascular thrombectomy in acute ischemic stroke treated with anticoagulants: a systematic review and meta-analysis. Thromb J. 2022;20(1):35.
Almallouhi E, Al Kasab S, Hubbard Z, Bass EC, Porto G, Alawieh A, et al. Outcomes of mechanical thrombectomy for patients with stroke presenting with low alberta stroke program early computed tomography score in the early and extended window. JAMA Netw Open. 2021;4(12):e2137708.
Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023.
Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023.
Volny O, Zerna C, Tomek A, Bar M, Rocek M, Padr R, et al. Thrombectomy vs medical management in low NIHSS acute anterior circulation stroke. Neurology. 2020;95(24):e3364–72.
Kim BJ, Menon BK, Yoo J, Han JH, Kim BJ, Kim CK, et al. Effectiveness and safety of EVT in patients with acute LVO and low NIHSS. Front Neurol. 2022;13:955725.
Feil K, Matusevicius M, Herzberg M, Tiedt S, Kupper C, Wischmann J, et al. Minor stroke in large vessel occlusion: a matched analysis of patients from the German Stroke Registry-Endovascular Treatment (GSR-ET) and patients from the Safe Implementation of Treatments in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Eur J Neurol. 2022;29(6):1619–29.
Nogueira RG. Endovascular therapy for low NIHSS ischemic strokes. 2019. https://ClinicalTrials.gov/show/NCT04167527. Accessed 18 Oct 2022.
Yeo LLL, Jing M, Bhogal P, Tu T, Gopinathan A, Yang C, et al. Evidence-based updates to thrombectomy: targets, new techniques, and devices. Front Neurol. 2021;12:712527.
NINDS. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7.
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29.
English JD, Yavagal DR, Gupta R, Janardhan V, Zaidat OO, Xavier AR, et al. Mechanical thrombectomy-ready comprehensive stroke center requirements and endovascular stroke systems of care: recommendations from the endovascular stroke standards committee of the society of vascular and interventional neurology (SVIN). Interv Neurol. 2016;4(3–4):138–50.
Masoud HE, de Havenon A, Castonguay AC, Asif KS, Nguyen TN, Mehta B, et al. 2022 Brief Practice update on intravenous thrombolysis before thrombectomy in patients with large vessel occlusion acute ischemic stroke: a statement from society of vascular and interventional neurology guidelines and practice standards (GAPS) committee. Stroke Vasc Interv Neurol. 2022;2(4):e000276.
Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325(3):244–53.
LeCouffe NE, Kappelhof M, Treurniet KM, Rinkel LA, Bruggeman AE, Berkhemer OA, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833–44.
Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981–93.
Meinel TR, Kaesmacher J, Buetikofer L, Strbian D, Eker OF, Cognard C, et al. Time to treatment with bridging intravenous alteplase before endovascular treatment:subanalysis of the randomized controlled SWIFT-DIRECT trial. J Neurointerv Surg. 2022.
Mitchell PJ, Yan B, Churilov L, Dowling RJ, Bush SJ, Bivard A, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4.5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet. 2022;400(10346):116–25.
Roaldsen MB, Jusufovic M, Berge E, Lindekleiv H. Endovascular thrombectomy and intra-arterial interventions for acute ischaemic stroke. Cochrane Database Syst Rev. 2021;6:CD007574.
Renu A, Millan M, San Roman L, Blasco J, Marti-Fabregas J, Terceno M, et al. Effect of intra-arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA. 2022;327(9):826–35.
Huang X, MacIsaac R, Thompson JL, Levin B, Buchsbaum R, Haley EC Jr, et al. Tenecteplase versus alteplase in stroke thrombolysis: an individual patient data meta-analysis of randomized controlled trials. Int J Stroke. 2016;11(5):534–43.
Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161–9.
Zhu A, Rajendram P, Tseng E, Coutts SB, Yu AYX. Alteplase or tenecteplase for thrombolysis in ischemic stroke: an illustrated review. Res Pract Thromb Haemost. 2022;6(6):e12795.
Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573–82.
Bivard A, Zhao H, Churilov L, Campbell BCV, Coote S, Yassi N, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol. 2022;21(6):520–7.
Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet. 2023;401(10377):645–54.
Genentech I. Tenecteplase in stroke patients between 4.5 and 24 hours. 2018. https://ClinicalTrials.gov/show/NCT03785678. Accessed 18 Oct 2022.
Hill M. A randomized controlled trial of TNK-tPA versus standard of care for minor ischemic stroke with proven occlusion. 2015. https://ClinicalTrials.gov/show/NCT02398656. Accessed 18 Oct 2022.
Coutts SB, Dubuc V, Mandzia J, Kenney C, Demchuk AM, Smith EE, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015;46(3):769–74.
Adeoye OM. Multi-arm optimization of stroke thrombolysis. 2018. https://ClinicalTrials.gov/show/NCT03735979. Accessed 18 Oct 2022.
Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–5.
Xie Y, Oppenheim C, Guillemin F, Gautheron V, Gory B, Raoult H, et al. Pretreatment lesional volume impacts clinical outcome and thrombectomy efficacy. Ann Neurol. 2018;83(1):178–85.
Baron JC. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat Rev Neurol. 2018;14(6):325–37.
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97.
Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7(5):378–85.
Felberg RA, Burgin WS, Grotta JC. Neuroprotection and the ischemic cascade. CNS Spectr. 2000;5(3):52–8.
Fisher M, Savitz SI. Pharmacological brain cytoprotection in acute ischaemic stroke - renewed hope in the reperfusion era. Nat Rev Neurol. 2022;18(4):193–202.
Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, et al. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci. 2007;27(37):9901–15.
Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284(5421):1845–8.
Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke. 2015;10(2):143–52.
Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S52–7.
Piccardi B, Arba F, Nesi M, Palumbo V, Nencini P, Giusti B, et al. Reperfusion injury after ischemic stroke study (RISKS): single-centre (Florence, Italy), prospective observational protocol study. BMJ Open. 2018;8(5):e021183.
Suzuki Y, Nagai N, Umemura K. A review of the mechanisms of blood-brain barrier permeability by tissue-type plasminogen activator treatment for cerebral ischemia. Front Cell Neurosci. 2016;10:2.
Inzitari D, Giusti B, Nencini P, Gori AM, Nesi M, Palumbo V, et al. MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke. 2013;44(10):2901–3.
Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56.
Takamatsu H, Tsukada H, Kakiuchi T, Nishiyama S, Noda A, Umemura K. Detection of reperfusion injury using PET in a monkey model of cerebral ischemia. J Nucl Med. 2000;41(8):1409–16.
Ames A III, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52(2):437–53.
Crowell RM, Olsson Y. Impaired microvascular filling after focal cerebral ischemia in the monkey. Modification by treatment. Neurology. 1972;22(5):500–4.
Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol. 2018;315(3):H550–62.
Xiong XY, Liu L, Yang QW. Refocusing neuroprotection in cerebral reperfusion era: new challenges and strategies. Front Neurol. 2018;9:249.
Puig J, Shankar J, Liebeskind D, Terceno M, Nael K, Demchuk AM, et al. From time is brain” to “imaging is brain: a paradigm shift in the management of acute ischemic stroke. J Neuroimaging. 2020;30(5):562–71.
Farzin B, Fahed R, Guilbert F, Poppe AY, Daneault N, Durocher AP, et al. Early CT changes in patients admitted for thrombectomy: intrarater and interrater agreement. Neurology. 2016;87(3):249–56.
Sheth SA, Liebeskind DS. Imaging evaluation of collaterals in the brain: physiology and clinical translation. Curr Radiol Rep. 2014;2(1):29.
Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33(8):1168–72.
Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1–2):53–68.
El Amki M, Gluck C, Binder N, Middleham W, Wyss MT, Weiss T, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. 2020;33(2):108260.
Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44(11):3202–10.
Schuhmann MK, Krstic M, Kleinschnitz C, Fluri F. Fingolimod (FTY720) Reduces cortical infarction and neurological deficits during ischemic stroke through potential maintenance of microvascular patency. Curr Neurovasc Res. 2016;13(4):277–82.
Tian DC, Shi K, Zhu Z, Yao J, Yang X, Su L, et al. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann Neurol. 2018;84(5):717–28.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–202.
van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130(Pt 12):3063–74.
Kuczynski AM, Marzoughi S, Al Sultan AS, Colbourne F, Menon BK, van Es A, et al. Therapeutic hypothermia in acute ischemic stroke-a systematic review and meta-analysis. Curr Neurol Neurosci Rep. 2020;20(5):13.
Zhao H, Steinberg G. Limited therapeutic time windows of mild-to-moderate hypothermia in a focal ischemia model in rat. Stroke Res Treat. 2011;2011:131834.
Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878–87.
Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39(9):2544–53.
Tymianski M. Combining neuroprotection with endovascular treatment of acute stroke: is there hope? Stroke. 2017;48(6):1700–5.
Hill MD, Martin RH, Mikulis D, Wong JH, Silver FL, Terbrugge KG, et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11(11):942–50.
Mayor-Nunez D, Ji Z, Sun X, Teves L, Garman JD, Tymianski M. Plasmin-resistant PSD-95 inhibitors resolve effect-modifying drug-drug interactions between alteplase and nerinetide in acute stroke. Sci Transl Med. 2021;13(588).
Hernandez-Enriquez M, Abad-Santos F, Cotgreave I, Gallego J Sr, Jilma B, Flores AF, et al., editors. LB2 - April: a double-blind, placebo-controlled, randomized, phase Ib/IIa clinical study of aptoll for the treatment of acute ischemic stroke. International Stroke Conference; 2023 February 8, 2023; Dallas, Texas.
Hong JM, Lee JS, Lee YB, Shin DH, Shin DI, Hwang YH, et al. Nelonemdaz for patients with acute ischemic stroke undergoing endovascular reperfusion therapy: a randomized phase II trial. Stroke. 2022;53(11):3250–9.
Li W, Qi Z, Ma Q, Ding J, Wu C, Song H, et al. Normobaric hyperoxia combined with endovascular treatment for patients with acute ischemic stroke: a randomized controlled clinical trial. Neurology. 2022;99(8):e824–34.
Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Marti-Fabregas J, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453–60.
Sansing L, editor. Primary results of the stroke prediction assessment network. International Stroke Conference; 2023 February 8, 2023; Dallas, Texas.
Chamorro A, Lo EH, Renu A, van Leyen K, Lyden PD. The future of neuroprotection in stroke. J Neurol Neurosurg Psychiatry. 2021;92(2):129–35.
Savitz SI, Baron JC, Fisher M, STAIR X Consortium. Stroke treatment academic industry roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke. 2019;50(4):1026–31.
Rocha M, Desai SM, Jadhav AP, Jovin TG. Prevalence and temporal distribution of fast and slow progressors of infarct growth in large vessel occlusion stroke. Stroke. 2019;50(8):2238–40.
Wu TC, Sarraj A, Jacobs A, Shen L, Indupuru H, Biscamp D, et al. Telemedicine-guided remote enrollment of patients into an acute stroke trial. Ann Clin Transl Neurol. 2015;2(1):38–42.
Cooray C, Fekete K, Mikulik R, Lees KR, Wahlgren N, Ahmed N. Threshold for NIH stroke scale in predicting vessel occlusion and functional outcome after stroke thrombolysis. Int J Stroke. 2015;10(6):822–9.
Sergot PB, Maza AJ, Derrick BJ, Smith LM, Berti LT, Wilcox MR, et al. Portable Neuromonitoring device detects large vessel occlusion in suspected acute ischemic stroke. Stroke. 2021;52(4):1437–40.
Ebinger M, Siegerink B, Kunz A, Wendt M, Weber JE, Schwabauer E, et al. Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA. 2021;325(5):454–66.
Kerr DM, Fulton RL, Lees KR, Collaborators V. Seven-day NIHSS is a sensitive outcome measure for exploratory clinical trials in acute stroke: evidence from the Virtual International Stroke Trials Archive. Stroke. 2012;43(5):1401–3.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajendram, P., Ikram, A. & Fisher, M. Combined Therapeutics: Future Opportunities for Co-therapy with Thrombectomy. Neurotherapeutics 20, 693–704 (2023). https://doi.org/10.1007/s13311-023-01369-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01369-1