Abstract
Introduction
Basal insulin is widely recommended for the treatment of type 2 diabetes mellitus (T2DM) patients who are unable to achieve glycemic control with oral antidiabetic drug(s) (OADs). However, some patients are still unable to control their blood glucose levels even when on basal insulin-supported OAD(s) therapy (BOT). The aim of this study was to investigate the factor(s) predicting patient response to BOT.
Methods
A total of 212 patients with T2DM, ranging in age from 18 to 65 years, admitted to the university hospital of Sun Yat-sen University, Guangzhou, China, were enrolled in the study between January 2013 and July 2016. All patients had fasting blood glucose levels of ≥ 10.0 mmol/L despite receiving OAD(s) treatment. According to study design, these patients first received intensive insulin therapy for 2 weeks to attain and maintain their glycemic goals and then were switched to BOT. Responders were defined as subjects who maintained their glycemic targets with BOT for at least 3 months; all others were considered to be non-responders. The characteristics between responders and non-responders were compared.
Results
Compared with non-responders, responders had a shorter duration of diabetes (5.1 ± 5.0 vs. and 10.1 ± 3.2 years; P < 0.001) and a higher 2-h postprandial C-peptide-to-fasting C-peptide ratio (2 h-PCP/FCP: 1.95 ± 0.51 vs. 1.67 ± 0.32; P < 0.01). Responders showed a lower proportion of previous treatment with insulin (69/100 vs 40/3; P < 0.001) and sulfonlureas or glinides (116/50 vs 40/0; P <0.001) than non-responders. Multivariate logistic regression analysis showed that previous insulin treatment (odds ratio [OR] 17.677, 95% confidence interval [CI] 5.205–60.027; P < 0.001) and the 2 h-PCP/FCP ratio (OR 0.241, 95% CI 0.058–0.679; P = 0.007) had predictive value.
Conclusions
A higher 2 h-PCP/FCP ratio and a lack of previous insulin treatment increase the likelihood of BOT success.
Similar content being viewed by others
Introduction
Basal insulin is the recommended treatment for patients with type 2 diabetes mellitus (T2DM) who are unable to achieve their glycemic targets with oral antidiabetic drugs (OADs) [1,2,3]. However, in clinical practice, some patients still fail to achieve glycemic control after being started on basal insulin as an add-on therapy, necessitating a switch to other treatment schemes, such as a basal-bolus insulin regimen or injections with premixed insulin twice or three times per day. Such evidence implies the basal insulin-supported OAD(s) therapy (BOT) fails to effectively control glycemia in some T2DM patients. It has been reported that variables such as age, body mass index (BMI) and bedtime or post-breakfast plasma glucose levels may influence patient response to the treatment regimen [4]. For example, in insulin-naïve patients, BOT may be more appropriate for subjects with a lower BMI and higher post-breakfast plasma glucose levels [5]. In one study population of T2DM patients who switched from a premixed insulin regimen to BOT, the key factors most closely related to the efficacy of BOT were pre-treatment glycated hemoglobin (HbA1c) levels, duration of diabetes and postprandial C-peptide levels [6]. However, the above-mentioned studies focused on individuals who plan to switch from treatment with (an) OAD(s) alone to BOT. To date, it is unknown whether one or more of these factors may predict the response to BOT. However, the ability of the treating physician to identify those patients who will not respond to BOT before a new treatment is decided upon would reduce healthcare costs and save time. The aim of this study is to determine which factor(s) may predict patient response to BOT.
Methods
Subjects
All of the participants in this study were being treated for T2DM as inpatients in the Department of Endocrinology, the 3rd Affiliated Hospital, Sun Yat-sen University (Guangzhou, Peoples’ Republic of China) at the time of enrollment (January 2013 to July 2016). The age of the participants at enrollment ranged from 18 to 65 years. All had fasting blood glucose levels of ≥ 10.0 mmol/L, even though they were receiving OAD(s) therapy without insulin. The exclusion criteria were: (1) newly diagnosed or treated-naïve T2DM, (2) type 1 or secondary diabetes mellitus, (3) positive anti-glutamic acid decarboxylase antibody, (4) liver cirrhosis, (5) liver or renal dysfunction (i.e. serum alanine aminotransferase or aspartate aminotransferase levels were 2.5-fold higher than normal, and serum creatinine level was > 177 μmol/L), (6) systemic infection, (7) the use of corticosteroids and (8) pregnancy.
Study Design
All therapeutic regimens involving OAD(s) were discontinued at admission to the study. The patients were then placed on an intensive insulin therapeutic regimen (multiple daily insulin injections or continuous subcutaneous insulin infusion) to attain the pre-defined glycemic goal of a fasting capillary blood glucose level of ≤ 6.1 mmol/L and a capillary blood glucose level at 2 h after each of three meals of ≤ 8.0 mmol/L. This treatment regimen was maintained for 2 weeks after the glycemic goal was reached, at which time the patients were switched to BOT. Either glargine insulin or detemir insulin was chosen as the basal insulin. All OAD(s) currently available could be prescribed. Adjustments in therapy were determined by the treating endocrinologists based on their experiences. The patients were required to self-monitor their capillary blood glucose levels at least four times per week, which had to include the fasting glucose level and the glucose levels at 2 h after each of three meals. Patients were able to reach the specialists in charge at any time for consultations during the trial period. Participants who were able to maintain the glycemic target on the BOT regimen for at least 3 months were considered to be responders. Those participants who were unable to achieve or maintain the glycemic target on the BOT regimen were taken off this treatment by the treating physician and switched to a treatment of twice or multiple daily insulin injections with or without OAD(s); these individuals were considered to be non-responders.
Data Collection and Measurements
A medical history and physical examination were conducted at admission. On the second day, fasting blood samples were collected for measurement of fasting plasma C-peptide (FCP), HbA1c and fasting plasma glucose (FPG) levels and for other routine biochemical tests. Breakfast consisted of mixed meals for all patients. At 2 h after breakfast, blood samples were taken to measure the 2 h postprandial C-peptide (2 h-PCP) and postprandial plasma glucose (2 h-PPG) levels.
Routine clinical laboratory tests were performed using the Olympus AU640 auto-biochemistry analyzer (Olympus Corp., Tokyo, Japan). A magnetic antibody immunoassay was used to measure C-peptide levels (Beijing Bio-Ekon Biotechnology Co., Ltd., Beijing, China). HbA1c level was assayed using the Bio-Rad D-10™ high-pressure liquid chromatography system (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical Analysis
Data were analyzed using the PASW statistics 18.0 program (IBM Corp., Armonk, NY, USA). Continuous variables with a normal distribution were presented as the mean ± standard deviation; in all other cases, data were presented as the median and interquartile range. Differences between two groups were assessed by an independent t test or non-parametric test. A Chi-square test was performed to analyze the differences in rates between the two groups. A forward LR variable selection-based multivariate logistic regression was utilized to determine independent predictors. Pearson’s correlation and Spearman correlation were performed to analyze the relationships between variables. Statistical significance was set at P < 0.05.
Compliance with Ethics Guidelines
All procedures followed in this study were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. The protocol and informed consent document were approved by the research ethics board of the 3rd Affiliated Hospital of Sun Yat-sen University. All patients gave written informed consents. The trial was not registered since it was performed in one hospital and was a retrospective analysis.
Results
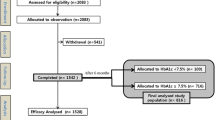
Of the 232 subjects screened, 221 patients received the intensive insulin treatment (11 patients withdrew before starting the intensive insulin treatment). Of these 221 patients, seven patients declined to go on the BOT therapeutic regimen and two patients were lost to follow-up. The final study population for analysis therefore consisted of 212 T2DM patients (Fig. 1). The characteristics of these 212 study subjects are shown in Table 1. A total of 169 (79.7%) patients achieved and maintained the target (i.e. responders) and 43 (20.3%) patients did not achieve or maintain the target (i.e. non-responders). Although non-responders were able to achieve and maintain the target of a fasting capillary blood glucose level of < 6.1 mmol/L, they were unable to achieve or maintain the target postprandial capillary blood glucose level of ≤ 8.0 mmol/L. The duration of diabetes, the percentage of patients with previous insulin, sulfonylurea or glinide treatments and the 2 h-PCP/FCP ratio were significantly different between responders and non-responders. These four variables were entered into the multivariate logistic regression analysis, and the results suggested that previous insulin treatment and the 2 h-PCP/FCP ratio had predictive value in terms of the success of BOT (Table 2). Spearman correlation analysis determined the coefficient between the duration of diabetes and the 2 h-PCP/FCP ratio to be − 0.223 (P < 0.001).
Discussion
The results of our study indicate that the 2 h-PCP/FCP ratio and previous insulin treatment can predict the success of BOT. More specifically, a high 2 h-PCP/FCP ratio and the absence of previous insulin treatment in a patient’s medical history suggest a higher success rate of BOT. In addition, the duration of diabetes had a close correlation with 2 h-PCP/FCP. Consequently, given the convenience of this parameter, the duration of diabetes may be a promising surrogate predictor.
Clinical experience suggests that individuals with the same disease can differ in their responses to the same treatments [7]. Therefore, predictors of therapeutic response are of great help to the treating physician in deciding upon a treatment program. The UK Prospective Diabetes Study has shown that most patients with T2DM will require treatment with exogenous insulin at some point during their lifetime [8, 9]. However, insulin is withheld from many patients due to a variety of reasons, including concerns about injection pain [10] and lifestyle restrictions [11]. Studies has shown that patients significantly value reducing the number of insulin injections [12]. In addition, increased injection frequency has been associated with poor adherence to the protocol, with one study showing that adherence was 78.3% in patients requiring one injection per day and 60.8% in patients requiring four injections per day (P < 0.0001) [13]. BOT requires only one single injection per day, which reduces patient reluctance to insulin therapy and increases compliance to the regimen. Thus, it is of great practical value to identify the predictor(s) of patient response to BOT.
Both BOT and other therapeutic regimens, which in this study included twice or multiple daily insulin injections with or without OAD(s), provide basal insulin to the patient. Therefore, as expected, both the FCP levels and the FCP/FPG ratios were similar between two groups (responders and non-responders) in our study. The main distinction between BOT and other regimens is that BOT cannot imitate endogenous stimulated insulin secretion, while other regimens can. Stimulated insulin secretion contributes significantly to the postprandial blood glucose level, as confirmed in our study in which all non-responders had to discontinue BOT because the target postprandial blood glucose level could not be achieved. This result suggests that the indictor(s) which reflect(s) stimulated insulin secretion would be predictor(s) of BOT response.
Various indices have been reported to reflect stimulated insulin secretion and to act as predictors of insulin therapy, such as first-phase and second-phase insulin secretion [14], glucagon loading serum insulin and C-peptide level, and fasting and 2 h-PCP level alone or adjusted by corresponding glucose levels [6, 15,16,17,18,19]. The serum C-peptide level reflects endogenous insulin secretion more directly than does the serum insulin level. Moreover, the serum C-peptide level can be used to assess beta-cell function even in patients undergoing insulin therapy. Thus, for our study, we selected serum C-peptide rather than insulin as the relevant parameter. In a number of previous studies, the ratio of C-peptide adjusted by glucose was superior to C-peptide alone [15,16,17,18]. We found, however, that neither 2 h-PCP alone nor the ratio of 2 h-PCP adjusted by glucose differed between the two groups. This discrepancy between our study and previous ones [15,16,17,18] is presumably due to sampling error and target differences. The distributions of both the FCP/FPG and 2 h-PCP/2 h-PPG ratios were skewed in our study, whereas they showed a normal distribution in the above-mentioned studies [15,16,17,18]. One explanation for this difference in distribution may be the different sampling times in these studies. In previous studies, blood samples were collected after or during a period of intensive insulin therapy, while in our study, they were collected prior to the patients starting on intensive insulin therapy. A second explanation may be differences in the target. The previous studies targeted the requirement for insulin therapy, whereas our study targeted the response to BOT. Notably, we found a significant difference in the 2 h-PCP/FCP ratio between the responders and non-responders, and its predictive value was further supported by the results of the multivariate logistic regression. Why was the 2 h-PCP/FCP ratio superior to 2 h-PCP alone or adjusted by glucose? The answer may be partly attributed to the C-peptide measurement. It is possible that factors are present in the C-peptide measurement which may be very influential [20] and that this interference is attenuated by adjusting the 2 h-PCP level by the fasting C-peptide level.
The duration of diabetes in responders was significantly shorter than that in non-responders, but the multivariate logistic regression did not suggest that this variable was a predictor of BOT success. The explanation for this difference is that beta-cell function was more direct than the duration of diabetes. Therefore, the duration of diabetes was removed in the multivariate logistic regression. It has been proven that beta-cell function progressively deteriorates over time [8, 21, 22]. In line with those previous studies, we also found that the 2 h-PCP/FCP ratio had a tight correlation with the duration of diabetes. Thus, considering the convenience of the duration of diabetes as a predictor of treatment success, this variable is worth further study.
Compared with the non-responder group, the proportion of patients who had received insulin treatment prior to this study was lower in the responder group. The multivariate logistic regression also suggested that a longer previous treatment with insulin reduced the response to BOT. This finding seems contrary to earlier reported results showing insulin therapy to be beneficial to the protection and preservation of beta-cell function. In fact, these results are not contradictory. These previous studies focused on the subsequent effects of the various treatment options while we focused on whether the patients had received insulin treatment prior to the study. In general, more previous insulin treatment in patients’ history corresponds with a longer duration of diabetes and worse beta-cell function.
The difference between the responders and non-responders in terms of previous sulfonylurea or glinide treatments was also significant. However, this variable was removed in the multivariate logistic regression. Like previous insulin treatment, as discussed above, the results did not suggest that treatment with sulfonylureas or glinides deteriorated beta-cell function; rather, previous intake of sulfonylurea or glinide drugs corresponded with a longer duration of diabetes.
Pre-treatment HbA1c level has been reported to be one of the key factors closely related to the efficacy of BOT [6]. However, in our study it was similar in both responders and non-responders. This discrepancy is attributed to the differences in patients enrolled in these two studies and to differences in the protocols. In the earlier study, the patients were treated twice daily with premixed 30R insulin with or without OAD(s) prior to initiation of the study and then randomized into either BOT or premixed 30R insulin twice daily plus OAD(s) treatment group. In contrast, in our study these pre-treatment options were not required, and all patients received intensive insulin therapy for 2 weeks, followed by a switch to BOT.
In our study, several factors possibly limit the extent to which these results can be generalized. First, the range of FPG level (≥ 10.0 mmol/L) and age (18–65 years), regardless of pre-treatment options and duration of diabetes, were quite wide. Hence, individual variation may be large, and some characteristics may be obscured. However, this scenario is closer to reality and is more valuable to clinical practice. Second, modification of the therapeutic regimen was at the discretion of the treating physician, based on personal experiences, which could lead to bias. Third, there were far fewer non-responders than responders, which may have obscured some characteristics or effects of the treatment.
Conclusions
The results of our study suggest that both the 2 h-PCP/FCP ratio and previous insulin treatment are predictors of response to BOT. A higher 2 h-PCP/FCP ratio and the lack of previous insulin treatment correspond with a higher likelihood of BOT success. The duration of diabetes is a promising predictor that requires further study.
References
Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13[Suppl 1]:1–68.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement–executive summary. Endocr Pract. 2013;19:536–57.
Lavernia F. What options are available when considering starting insulin: premix or basal? Diabetes Technol Ther. 2011;13[Suppl 1]:S85–92.
Fonseca V, Davidson J, Home P, Snyder J, Jellinger P, Dyhr TA, et al. Starting insulin therapy with basal insulin analog or premix insulin analog in T2DM: a pooled analysis of treat-to-target trials. Curr Med Res Opin. 2010;26:1621–8.
Bu S, Guo XH, Yang WY, Lu GZ, Yang ZJ, Ren TT, et al. Post-hoc analyses of type 2 diabetes patients switch from premixed insulin regimen to basal insulin plus oral hypoglycemic agents regimen. Zhonghua Yi Xue Za Zhi. 2007;87:3115–8.
Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and beta-adrenergic blockers?. A systematic review. BMC Med. 2013;11:141.
[No authors listed] Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53
Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330–6.
Cefalu WT. Evaluation of alternative strategies for optimizing glycemia: progress to date. Am J Med. 2002;113[Suppl 6A]:23S–35S.
Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543–5.
Hauber AB, Johnson FR, Sauriol L, Lescrauwaet B. Risking health to avoid injections: preferences of Canadians with type 2 diabetes. Diabetes Care. 2005;28:2243–5.
Donnelly LA, Morris AD, Evans JM. Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM. 2007;100:345–50.
Lin JD. Levels of the first-phase insulin secretion deficiency as a predictor for type 2 diabetes onset by using clinical-metabolic models. Ann Saudi Med. 2015;35:138–45.
Fujiwara D, Takahashi K, Suzuki T, Shii M, Nakashima Y, Takekawa S, et al. Postprandial serum C-peptide value is the optimal index to identify patients with non-obese type 2 diabetes who require multiple daily insulin injection: analysis of C-peptide values before and after short-term intensive insulin therapy. J Diabetes Investig. 2013;4:618–25.
Saisho Y, Kou K, Tanaka K, Abe T, Shimada A, Kawai T, et al. Postprandial serum C-peptide to plasma glucose ratio predicts future insulin therapy in Japanese patients with type 2 diabetes. Acta Diabetol. 2013;50:987–8.
Saisho Y, Kou K, Tanaka K, Abe T, Kurosawa H, Shimada A, et al. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58:315–22.
Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, et al. Utility of indices using C-peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2:297–303.
Goto A, Takaichi M, Kishimoto M, Takahashi Y, Kajio H, Shimbo T, et al. Body mass index, fasting plasma glucose levels, and C-peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr J. 2010;57:237–44.
Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803–17.
Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over 6 years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998;15:297–303.
[No authors listed] Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
Acknowledgements
Funding
This study and article processing charges for this publication were funded by National Key R&D Program of China (2017YFA0105803), the General Program of National Natural Science Foundation of China (81770826), the 5010 Clinical Research Projects of Sun Yat-sen University (2015015), the Science and Technology Plan Projects of Guangdong Province (2016A050502010), the Key Special Projects of Medical and Health Collaborative Innovation of Guangzhou City (201604020016), and the Special Scientific Research Project of Guangzhou City (2060404).
Editorial Assistance
We thank statistician Lian-xiong Yuan for his advice and help in the statistical analysis. We also thank the American Journal Experts Co. for language revision during the writing of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Giving Thanks
We would like to thank all of the doctors, nurses, technicians and patients involved for their dedication to this study.
Disclosures
All named authors (Pan-wei Mu, De-zhao Liu, Ying Lin, Dong Liu, Fan Zhang, Yong-jun Zhang, Shuo Lin, Lin-qin Wang, Man-man Wang, Jiong Shu, Long-yi Zeng and Yan-ming Chen) declare that they have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. The protocol and informed consent document were approved by the research ethics board of the 3rd Affiliated Hospital of Sun Yat-sen University. All patients gave written informed consent. The trial was not registered since it was performed in one hospital and was a retrospective analysis.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.5947846.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mu, Pw., Liu, Dz., Lin, Y. et al. The Postprandial-to-Fasting Serum C-Peptide Ratio is a Predictor of Response to Basal Insulin-Supported Oral Antidiabetic Drug(s) Therapy: A Retrospective Analysis. Diabetes Ther 9, 963–971 (2018). https://doi.org/10.1007/s13300-018-0404-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0404-6