Abstract
Background
Clinicians are encouraged to take an individualized approach when treatinghypertension in patients of African ancestry, but little is known about whythe individual patient may respond well to calcium blockers and diuretics,but generally has an attenuated response to drugs inhibiting therenin-angiotensin system and to β-adrenergic blockers. Therefore, wesystematically reviewed the factors associated with the differential drugresponse of patients of African ancestry to antihypertensive drugtherapy.
Methods
Using the methodology of the systematic reviews narrative synthesis approach,we sought for published or unpublished studies that could explain thedifferential clinical efficacy of antihypertensive drugs in patients ofAfrican ancestry. PUBMED, EMBASE, LILACS, African Index Medicus and the Foodand Drug Administration and European Medicines Agency databases weresearched without language restriction from their inception through June2012.
Results
We retrieved 3,763 papers, and included 72 reports that mainly considered the4 major classes of antihypertensive drugs, calcium blockers, diuretics,drugs that interfere with the renin-angiotensin system and β-adrenergicblockers. Pharmacokinetics, plasma renin and genetic polymorphisms did notwell predict the response of patients of African ancestry toantihypertensive drugs. An emerging view that low nitric oxide and highcreatine kinase may explain individual responses to antihypertensive drugsunites previous observations, but currently clinical data are verylimited.
Conclusion
Available data are inconclusive regarding why patients of African ancestrydisplay the typical response to antihypertensive drugs. In lieu ofbiochemical or pharmacogenomic parameters, self-defined African ancestryseems the best available predictor of individual responses toantihypertensive drugs.
Similar content being viewed by others
Background
There is a great need for individual treatment options in hypertensive patients ofAfrican ethno-geographical ancestry [1–5]. Compared with hypertension in other population subgroups, the disorderin these patients is often more severe, more resistant to treatment, and leads toearlier end organ damage and premature death [1–4]. Thus, hypertension seems to be a more aggressive disease in patients ofAfrican ancestry. This has important implications for the choice of anantihypertensive agent [3, 5].
Antihypertensive drugs were the first cardiovascular therapy for which there was widerecognition of differences in clinical efficacy related to ethno-geographicalancestry [6]. Patients of African ancestry as a group respond better to calciumblockers and diuretics, while the response to β-adrenergic blockade andinhibition of the angiotensin converting enzyme is attenuated (Table 1) [3, 5, 7]. However, there is considerable interindividual variation in thisresponse [7, 8].
Greater knowledge about the potential causes for these differences might lead to moreindividualized treatment regimens, but to our knowledge, no previous study hassystematically addressed why patients of African ancestry may have this specificpattern of responses. The aim of this paper is to provide a systematic overview ofthe factors associated with the differential drug response of patients of Africanancestry to antihypertensive drug therapy.
Methods
We sought to identify all published or unpublished studies that considered potentialexplanations for the differential clinical efficacy of different classes ofantihypertensive drugs, used as single drug or single drug-based treatment innon-pregnant adults of sub-Saharan African descent with uncomplicated hypertension,defined as the absence of reported clinical heart failure, current stroke or endstage renal disease.
We first identified potential causes for differences in specific drug responses basedon ethno-geographic origin (Table 2). As we sought toexplain differential blood pressure lowering responses to different types ofantihypertensive drugs, we excluded general factors such as access to care anddifferences in socio-economic status. To answer the clinical question, why there wasa difference in response between people of African vs European ancestry, weconsidered pharmacokinetic variations including polymorphisms in cytochrome P450family of enzymes involved in phase I drug metabolism, and polymorphisms in genesencoding enzymes involved in phase II drug metabolism. Furthermore, we consideredgenetic polymorphisms that may influence pharmacodynamics including alpha-adducin(ADD1), subunits of G-proteins (GNB3 and GNAS1), theβ-1-adrenergic receptor (ADRB1), endothelial nitric oxide synthase(NOS3), and components of the renin-angiotensin-aldosterone (RAAS)system, angiotensinogen (AGT), renin (REN), angiotensin convertingenzyme (ACE), the angiotensin II receptor type I (AGTR1 orAT1R), and aldosterone synthase (CYP11B2) [9]. Finally, hypertension in persons of African ancestry is characterized byhigh vascular contractility, greater salt sensitivity and, in general, low plasmarenin activity [2], and the molecular basis of these changes has been related to low nitricoxide (NO) bioavailability [10], to the activity of Ca2+ATPase, myosin ATPase,Na+K+ ATPase, and to the central regulatory enzyme ofenergy metabolism, creatine kinase (CK), which rapidly regenerates adenosinetriphosphate (ATP) from phosphocreatine near these ATPases [11, 12].
Using these environmental, pharmacokinetic, pharmacodynamic and pharmacogenomicfactors, we conducted a systematic literature search in electronic databases,including PUBMED, EMBASE, LILACS (Literatura Latino-Americana y del Caribe enCiencias de la Salud), the African Index Medicus (AIM), and the Food and DrugAdministration (FDA) and European Medicines Agency (EMA) databases, dated June2012.
We developed a search strategy to find papers that considered causes for differentialresponses, rather than finding clinical trials per se[3]. To reach this end, the most effective strategy in terms of the yield ineligible papers was to not include drug names, or “hypertension”, butthe factors as mentioned in Table 2, using the followingkeywords: “(salt OR pharmacokinetic OR resorption OR bioavailability OR liverOR first pass OR metabolism OR cytochrome OR n-acetyltransferase ORcatechol-o-methyltransferase OR phenol sulfotransferase OR distribution OR proteinbinding OR elimination OR pharmacodynamic OR pharmacogenetic OR receptor ORG-protein OR alpha-adducin OR nitric oxide OR c-GMP OR cAMP OR sarcoendoplasmic ORcalcium OR ion OR creatine kinase OR rho kinase OR “myosine ATPase” OR“myosin light chain kinase”) and (black* OR Afr* OR Creole OR CarribeanOR Caribbean OR negr* OR ethnic*) and antihypertensive.”
Finally, we hand-searched for studies by using electronic cross referencing(“related citations”) from PUBMED, references from textbooks, narrativereviews and systematic reviews; by contacting experts; and by searching theInternet. We did not restrict the searches to any specific language.
To produce a rigorously conducted narrative systematic review, we used the“narrative synthesis approach” (the PRISMA guideline is not designed fornarrative systematic reviews) [13]. This recently developed methodology is applied when one expectsconsiderable heterogeneity among the studies of interest. Distinctively, a narrativerather than a statistical summary of the findings of studies is used to perform thedata synthesis, which yields a more detailed analysis of heterogeneous data withless loss of information [13].
Any experimental research that is reported in the manuscript has been performed withthe approval of an appropriate ethics committee. Research carried out on humans werein compliance with the Helsinki Declaration, and experimental research on animalsfollowed internationally recognized guidelines.
Results
Paper flow
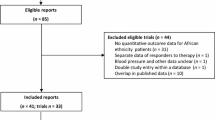
We retrieved 2,520 citations in PUBMED, 1,002 in EMBASE, 4 in LILACS, 2 in theAIM, 2 in the FDA and 229 in the EMA database for a total of 3,759 citations.Four citations in the EMA database contained 2 clustered reports, adding 4papers to yield a total of 3,763 papers. After removing duplicate reports andapplying the inclusion criteria, 55 papers were included from the electronicsearches (please see Paper Flow, Figure 1, withdetailed mention of the reason for exclusion) [14–68]. EMBASE, LILACS, AIM and the FDA database did not yield anyadditional included reports beyond the papers included from PUBMED, but oneadditional paper was included from the EMA database [68]. The majority of the excluded reports did not provide an explanationfor differences in antihypertensive drug response related to ancestry. Handsearch yielded 17 more papers [12, 69–84], most of which had no ancestry/ethnicity/race tag in PUBMED, or werenot indexed, such that these could not be retrieved with electronic searches. Wedid not use language restriction, but all included papers were written in theEnglish language.
Flow diagram. Data were retrieved from PUBMED, EMBASE, LILACS, theAfrican Index Medicus, and the Food and Drug Administration and EuropeanMedicines Agency databases. *Studies were excluded using a hierarchicalapproach. First, we excluded reports that did not fulfill the maininclusion criteria (n = 2,644): an original reportconsidering drug therapy with different available drug types innon-pregnant adults of African ancestry with uncomplicated hypertension,defined as the absence of clinical heart failure, stroke or end stagerenal disease as reported by the authors. Studies conducted exclusivelyin diabetics were also excluded in this step. Of the remaining studiesfulfilling these main inclusion criteria (n = 1,119), moststudies were excluded in the next step (n = 982), becausethese were not original reports providing an explanation for thedifference in response to antihypertensive drugs between ancestrygroups. As a quality and consistency check, each paper retrieved fromthe search yield (n = 3,763) was categorized, per database,thus the excluded paper categories harbor duplicate reports, occurringin more than one database. † Eligible reports thusfulfilled the inclusion criteria, and were original reports consideringpotential causes for the differential response of patients of Africanancestry to antihypertensive drugs used as single drug or singledrug-based treatment. Included studies from the electronic searches(n = 55) [14–68], and hand search (n = 17) [12, 69–84] are described in detail in the Results section.
Description of included studies
The included studies were original reports that provided, or attempted toprovide, an explanation for the differences in antihypertensive drug responsesbetween hypertensive patients of African and European ancestry. The design ofthe included studies varied, from observational studies to small and large scaleclinical trials, in subjects of sub-Saharan African descent, studied withinAfrica (Nigeria [46, 59, 67], Kenya [64], and South Africa [26, 61, 62, 69, 78]), or in the African diaspora (the Netherlands, persons from Suriname,the Dutch Antilles, and Ghana [12, 72, 73]; United Kingdom, persons from Nigeria [54, 60], Sierra Leone [54], Zimbabwe [54], Zambia [54], Tanzania [54], or country of origin not stated [50]; all other were in the United States, except for one paper that didnot state the location of the study [34]). Ethno-geographic origin was either self-defined, or defined by theauthors of the reports, in the participants being of European or Africanancestry. Authors used different nomenclature for African descent, includingblack people, blacks, black race, black skinned people, African-Americans andAfro-Caribbeans; as well as for European descent, including white and Caucasian.We unified this to: ‘persons (or patients) of African ancestry’versus ‘European ancestry’, throughout this paper, as thisnomenclature captures concepts of genomic variation, biology or geographichistory [85]. The majority of papers retrieved considered the four major classesof antihypertensive drugs: calcium blockers, diuretics, drugs that interferewith the renin-angiotensin-aldosterone system, and β-adrenergic blockers(Table 3). Data are synthesized below [13].
Narrative synthesis
Calcium blockers
Clinical efficacy
Calcium blockers are with diuretics among the most effective classes ofdrugs to reduce blood pressure in patients of African ancestry [3, 7]. This drug type remains effective in all subgroups of sex,age and blood pressure strata, including high baseline diastolic bloodpressure (>/= 110 mm Hg). Side effects include headache and ankleedema [3, 7].
Environmental factors
Calcium antagonists manifest a more robust blood pressure loweringeffect, even in the setting of salt intake ad libitum or a highsodium intake, albeit at the expense of a higher drug dose [34, 40, 41, 43]. When controlled, sodium intake in the studies varied between40 to 100 mmol/day in low salt, and 190 to 300 mmol/day in high saltconditions [34, 41, 43]. With a high salt diet and isradipine, mean systolic bloodpressure (SD) in hypertensive patients of African ancestry(n = 42) was: placebo 155.2 (19.3) vs. isradipine 139.3(15.0) mm Hg; a difference of −15.9; and in patients of Europeanancestry (n = 92) placebo 156.9 (14.5) vs isradipine 142.1(13.0); a difference of −14.8. With low salt, systolic bloodpressure in patients of African ancestry was placebo 142.9 (17.0) vsisradipine 135.8 (15.6); a difference of −7.1; and in patients ofEuropean ancestry placebo 143.5 (14.6) vs isradipine 135.9 (12.3), adifference of −7.6 [40]. In addition, with high salt intake, the mean blood pressurelowering effect of calcium blockers exceeded the effect of ACEinhibitors in patients of African, but not of European ancestry [41].
Pharmacokinetics
Nifedipine clearance is reported to be lower in persons of Africanancestry, with a 150% greater area under the plasma concentration-timecurve; and a 79% higher elimination half-life [46], but no significant differences were found for nitrendipine [58].
Regarding genetic polymorphisms and pharmacokinetics, verapamil is acytochrome CYP3A substrate, and CYP3A5 is thought to convert cortisol to6 b-hydroxycortisol in the kidney, and to be associated withsalt-sensitive hypertension. In the CYP3A5 gene, the A4G (*3)and G4A (*6) polymorphisms result in severely decreased expression ofCYP3A5 enzyme relative to a normal functional allele (*1) [24]. These polymorphisms were studied in the InternationalVerapamil/trandolapril Study (INVEST) Genetic Substudy (INVEST-GENES),which included hypertensive subjects with coronary artery disease(n = 537; 43 of African ancestry). However, no associationwas found with the antihypertensive response to verapamil [24]. Amlodipine is also extensively metabolized in the liver,mainly by CYP3A4 and possibly CYP3A5. In the African-American Study ofKidney Disease and Hypertension (AASK), 1,094 self-identifiedAfrican-American men and women between 18 and 70 years, diagnosed withhypertensive kidney disease (glomerular filtration rate between 20 and65 ml/min per 1.73 m2), were randomized to amlodipine,ramipril or metoprolol, and a mean goal arterial blood pressure (MAP) ofeither 102 to 107 mm Hg (usual MAP goal) or ≤92 mm Hg (low MAPgoal) to assess the effect on the decline in kidney function. Of these,159 participants were analyzed for CYP3A4 and CYP3A5polymorphisms. Only women randomized to a usual MAP goal, and with an Aallele at CYP3A4 A392G, were more likely to reach a target MAPof 107 mm Hg (adjusted hazard ratio of AA/AG compared to GG: 3.41 (95%CI: 1.20 to 9.64; P = 0.02). Among participantsrandomized to a lower MAP goal, men and women with the C allele atCYP3A4 T16090C were more likely to reach the target MAP of107 mm Hg (adjusted hazard ratio 2.04 (95% CI 1.17 to 3.56;P = 0.01). CYP3A5 A6986G was notassociated with blood pressure response in this study [17].
Pharmacodynamics
Profiling using age and ancestry was shown to be superior to renin levelsin predicting the magnitude of the antihypertensive response todiltiazem [38]. Calcium blockers’ main effect is vasodilation througha direct effect on the smooth muscle layer of resistance arteries [31]. The drugs reach their effect through a reduction of theintracellular calcium concentration in smooth muscle, by the blockingL-subtype, voltage-sensitive, slow calcium channels in cell membranesand calcium outflow from the sarcoendoplasmic reticulum [3, 11, 93].
The high efficacy of calcium blockers in patients of African ancestrypoints to enhanced vascular smooth muscle contractility in this group [11, 12, 36, 37, 39, 72]. This is thought to be a result of a “doublejeopardy”: a lack of NO bioavailability [10, 12, 31, 36, 37, 72, 80], and related high activity of the enzyme CK [10, 12, 72] (Figure 2). CK fuelsCa2+ ATPase at the sarcoendoplasmic reticulum and,thereby, calcium uptake, as well the ATPases directly leading tovasoconstriction [12, 72]. Thus, high vascular CK increases vascular contractility, asa final cellular step [72]. Furthermore, the high creatine demand associated with highcreatine kinase might induce a relative lack of L-arginine and NO [12].
Modulators of vascular contractility. This is a schematicrepresentation of the main regulatory pathways of vascularsmooth muscle contraction, based on Brewster et al.[12, 72]. Creatine kinase (CK) is colocalized withCa2+ ATPase and myosin ATPase, and evidencesuggests the enzyme is also colocalized with myosin light chain(LC) kinase, to rapidly supply these enzymes with ATP usingcreatine phosphate (Creatine ~ P) [11, 12, 72, 88–90]. The guanidino compounds creatine and nitric oxide(NO) have a common precursor in L-Arginine [12]. NO, RhoA/Rho kinase, and calcium-dependent pathwaysare intracellular effectors of blood pressure-regulating systemsthat converge on metabolic processes fueled by CK [11, 12, 72, 88–91, 93–95]. CK is high in persons of African ancestry [11, 12, 72, 73], and this is thought to lead to greater contractilityof vascular smooth muscle [11, 12, 72]. Vascular contractile responses can be reducedthrough enhancing NO-dependent pathways, including with ACEinhibitor (ACE-i) or nebivolol-induced NO synthesis, or throughindirect inhibition of CK-dependent pathways, as with calciumblockers (CaB) or β-adrenergic agonists. Calcium blockersmay block the entry of calcium in the cell as well as theoutflow from the sarcoendoplasmic reticulum (SER) [93]. β-adrenergic agonists reduce contractilitymainly through inhibition of myosin light chain kinase [91]. β-adrenergic blockers antagonize thisbeneficial effect, which may help explain the more frequentoccurrence of blood pressure increase with β-blockers inpersons of African ancestry [3, 92], within the context of the greater vascularcontractility in this population subgroup [11, 12, 36, 37, 39, 72]. cGMP, guanosine cyclic3′,5′-(monophosphate); MLCP, myosin light chainphosphatase.
Therefore, the clinical efficacy of calcium blockers in patients ofAfrican ancestry may depend on the strong antagonistic effect of thedrug on the enhanced vascular contractility induced by high CK and lowNO (Figure 2), but there are no clinical datayet showing this to predict the response to calcium blockers.
Pharmacogenomic factors were studied in the AASK study, where G12269A,C17888T and G20037A ACE polymorphisms were not associated withblood pressure lowering in participants randomized to amlodipine(n = 159) [23]. In addition, in a study including patients of Africanancestry (n = 108), functional variants in the promoterregion of the angiotensinogen gene (−217G = > A and−20A = > C), which influence the transcription ofthe gene, did not predict the response to nifedipine [26].
Diuretics
Clinical efficacy
Diuretics are among the most effective blood pressure lowering drugs inpatients of African ancestry [3, 7], although there is evidence that with high baseline diastolicblood pressures, calcium blockers are more effective [3]. Furthermore, there are concerns regarding metabolic sideeffects, including abnormal glucose tolerance. This might be ofparticular importance to patients of African ancestry, who have a higherrisk of developing diabetes, and often need to start treatment at ayounger age [3].
Environmental factors
In the Trial of Antihypertensive Interventions and Management (TAIM), 692participants (224 of African ancestry) aged 21 to 65 years, withdiastolic blood pressure between 90 and 100 mm Hg and weight between110% and 160% of ideal weight were randomized into diet (usual, lowsodium-high potassium, weight loss) and drug (placebo, 25 mg/daychlorthalidone or 50 mg/day atenolol) groups resulting in nine diet plusdrug combinations. When comparing subjects randomized to chlorthalidone(n = 24) vs placebo (n = 26) for usual vslow-sodium diet, adding sodium restriction (mean 100 mmol/day) to thediuretic drug did not enhance the blood pressure lowering effect [70].
Pharmacokinetics
No differences were found in bioavailability or elimination ofhydrochlorothiazide between ancestry groups [33].
Pharmacodynamics
The activity of the renin-angiotensin-aldosterone system is thought to beinversely related to the blood pressure response to diuretics [30]. Therefore, renin profiling was used to predict the response tohydrochlorothiazide in six papers, 25 mg/d in 363 participants (152 ofAfrican ancestry), of the Pharmacogenomic Evaluation of AntihypertensiveResponses (PEAR) study; [15] 12.5 to 50 mg/d in a Veterans Administration study in 335subjects (152 of African ancestry); [38] 50 mg/d in 83 patients of African ancestry; [57] 50 to 200 mg/d in 212 participants (129 of African ancestry) inanother Veterans Administration study; [63] 100 mg/d in 61 patients of African ancestry; [65] and 100 mg/d (vs furosemide 80 mg/d) in 29 patients of Africanancestry [66].
Renin did not predict the response to hydrochlorothiazide monotherapy in fourstudies [57, 63, 65, 66], nor to furosemide (80 mg/d) [66], or spironolactone 100 to 400 mg/d [65]. In the PEAR study, the β ± SE forprediction of systolic blood pressure with renin was1.87 ± 0.90 (P = 0.04), with arelative contribution of African ancestry of−2.12 ± 1.47 (P = 0.15); [15] and regression models that included ancestry and age explainedsimilar [15] or greater [38] variation in blood pressure response than renin.
Persons of African ancestry are reported to have a greater tendency to retainsalt [2, 11, 96]. This is thought to be a primary renal mechanism, as theincreased Na+ retention does not appear to be secondary toincreased production of aldosterone, deoxycorticosterone, cortisol or18-hydroxycortisol [82]. The main mode of action of thiazide diuretics is to inhibitNa+Cl−-cotransporter activity in the renaldistal convoluted tubule, blocking sodium reabsorption across the luminalmembrane. All sodium absorption throughout the kidney is energetically andosmotically driven by the basolateral sodium pumpNa+K+ ATPase [86]. ATP generation to this sodium pump is supported by CK, which istightly bound near Na+K+ ATPase to rapidly regenerateATP in situ[87]. CK is thus thought to directly provide ATP for sodiumreabsorption [11, 87] (Figure 3).
Pharmacodynamics of thiazide diuretics. This is a schematicreproduction of the kidney distal convoluted tubule. Sodiumretention is driven by basolateral Na+K+ATPase throughout the kidney [86]. Creatine kinase (CK), reported to be high in persons ofAfrican ancestry [11, 12, 72, 73], is tightly bound near basolateralNa+K+ ATPase, where it rapidly regeneratesATP to facilitate sodium retention [87]. Enhanced sodium retention occurs more frequently inpersons of African ancestry [96]. Thiazide diuretics counteract this effect, albeitindirectly and partly, through inhibition of luminalNa+Cl− -cotransport.
The high CK activity in persons of African ancestry has been linked to thegreater tendency in this group to retain salt [11, 96]. Also, evidence was found for a more activeNa+K+ 2Cl−-cotransporter in the thick ascending limbin persons of African ancestry [71]. This might render drugs that counteract sodium retention as amode of action to be highly effective, but further data are needed tosubstantiate how differences in kidney function in persons of African vsEuropean ancestry may impact responses to antihypertensive drugs.
In a pharmocogenomic approach, the association between variation in fivestructural genes encoding components of RAAS and the effect of monotherapywith hydrochlorothiazide 25 mg daily for four weeks, was studied in 255hypertensive men and women of African and 246 European ancestry, includingAGT (angiotensinogen) G-6A, AT1R (angiotensin IIreceptor, type 1) A1166C, ACE Insertion/Deletion; CYP11B2(aldosterone synthase) C-344 T, and REN (renin) A7174G. Only inwomen of African ancestry, but not in men or patients of European ancestry,blood pressure lowering was greater with an increasing number ofAGT–6A alleles (−11.3 mm Hg for GG, −18.2 mm Hg for AG and−22.2 mm Hg for AA; P = 0.03); and ofAT1R 1166A alleles (−14 mm Hg for CC, −15.6 mm Hgfor AC and −22.5 mm Hg for AA; P = 0.04) [30].
The association between the response to hydrochlorothiazide 25 mg daily forfour weeks and a polymorphism (C825T) in exon 10 of the gene encoding theb3-subunit of G proteins (GNB3), which potentially leads toenhanced sodium-proton antiport activity was assessed in men and women ofAfrican (n = 197) and European ancestry (n = 190) [32]. Relative frequencies of the CC, TC and TT genotypes ofGNB3 differed significantly between groups, with the T allelesignificantly more frequent in patients of African than in European ancestry(76.1% versus 28.9%, P <0.01). In patients of European ancestry,and in men, but not in women of African ancestry, the presence of the Tallele was associated with a greater reduction in systolic and diastolicblood pressure with treatment, with mean declines of respectively, 10.2 and5.9 mm Hg in CC; 13.6 and 7.8 mm Hg in TC; and 16.3 and 10.5 mm Hg in TTgenotypes, accounting for 3.1% and 4.5%, respectively, of interindividualvariation in the systolic and diastolic blood pressure responses tohydrochlorothiazide [32].
The WNK-SPAK-Na+Cl−-cotransporter pathway hasbeen previously implicated in thiazide response, as variations inWNK1 were associated with differential BP response tohydrochlorothiazide [76]. Therefore, 195 “good” and 194 “poor”responders to hydrochlorothiazide 25 mg daily from the Genetic Epidemiologyof Responses to Antihypertensives study were genotyped for approximately 100single nucleotide polymorphisms within 5,000 bases of STK39, with areplication sample of 201 hydrochlorothiazide-treated hypertensives from thePEAR study. No polymorphism was significantly associated with blood pressureresponse [76].
In the Genetics of Hypertension-Associated Treatment Study (GenHAT),participants of the Antihypertensive and Lipid-Lowering treatment to preventHeart Attack Trial (ALLHAT) were studied for the association betweenα-adducin Gly460Trp polymorphism and blood pressure response tochlorthalidone vs other drugs (n = 36,913; 12,696 of Africanancestry). Carriers of the Trp allele are reported to have a greateroccurrence of salt-sensitive hypertension, and blood pressure response todiuretics was pronounced with the Trp allele, compared to the Gly allele inEuropean ancestry populations [25]. However, GlyGly homozygotes were significantly more frequent inparticipants of African ancestry than in other participants (82.6% vs 67.4%,P <0.01), and there was no significant difference insystolic or diastolic blood pressure response between Trp allele carriersand non-carriers (systolic/diastolic reduction in Trp allele carriers−7.42/–3.23 mm Hg, vs −7.44/–3.57 mm Hg innon-carriers; P >0.05) [25].
Also, polymorphisms of the GRK2 gene (ADRBK1) and GRK5Gln41Leu, which are reported to mediate down-regulation of β-adrenergicsignaling, were studied in 418 patients (167 of African ancestry) from thePEAR study. The genotypes were not associated with the blood pressureresponse to hydrochlorothiazide [77].
Finally, using genome wide analysis, good and poor responders tohydrochlorothiazide of African (n = 194) and European ancestry(n = 195) were compared. Variation in one region on chromosome12q15 emerged to be significantly associated with blood pressure response,but only in subjects of African ancestry [21]. Follow-up analysis favored YEATS4, a gene probablyencoding a transcription factor, over LYZ, encoding lysozyme, aspositional candidate genes [21]. The study has now been replicated [83], but the biological mechanism that may underlie the observedassociations with blood pressure response to hydrochlorothiazide is hithertounclear.
Inhibitors of the RAAS system
ACE inhibitors
Clinical efficacy
ACE inhibitors are known to induce less blood pressure lowering inpatients of African than in European ancestry [7]. In the former, ACE inhibitors do not differ from placebo inachieving diastolic goal blood pressure with monotherapy [3]. The main difference in side effects is the relatively highincidence of angioedema with the use of ACE inhibitors in patients ofAfrican ancestry [3].
Environmental effects
High salt intake reduces the blood pressure lowering efficacy of ACEinhibition. With a high salt diet (190 mmol sodium/day) and enalaprilstudied in 391 subjects (96 of African ancestry), systolic bloodpressure reduction in mm Hg (SD) was smaller in patients of African,than of European ancestry (respectively, placebo 156.5 (13.1) vs.enalapril 146.2 (16.4); difference −10.3 for African, and placebo159.2 (13.4) vs enalapril 144.2 (17.5); difference −15.0 forEuropean ancestry groups).
With low salt (88 mmol sodium/day), blood pressure was lower, but thedifference persisted (African ancestry, placebo 145.0 (16.1) vsenalapril 137.2 (19.2) difference −7.7; European ancestry placebo145.1 (17.1) vs enalapril 132.4 (16.2), difference −12.7) [41]. Drug efficacy of ACE inhibitors in patients of Africanancestry can thus be modulated by controlling salt intake, or addingthiazide diuretics to the drug regimen [8]. However, even with low salt, the blood pressure loweringeffect of ACE inhibitors is greater in patients of European ancestry [41]. This implies that other factors are involved in thedifference in drug response.
Pharmacokinetics
In the ramipril arm of the AASK study [17], there were no associations between CYP3A4 A392G,CYP3A4 T16090C or CYP3A5 A6986G genotypes and timeto reach target mean arterial pressure among men or women randomized toa low or usual mean arterial pressure.
Pharmacodynamics
The main mode of action of ACE inhibition is well known, the drugs reducethe activity of angiotensin converting enzyme, and eventually,angiotensin, aldosterone and salt retention. In addition, ACE inhibitorspromote NO synthesis in the endothelium [97].
A repressed RAAS system occurs with greater frequency in persons ofAfrican ancestry [2, 35]. Therefore, any drug further repressing this system could beexpected to be less effective in this population group [15, 22, 38, 50]. However, clinical trials have produced mixed results inwhether low renin levels adequately predict an attenuatedantihypertensive response [22, 38, 40, 50]. As with diuretics, profiling based on age and ancestry wasshown to be superior to renin levels in predicting the magnitude of theantihypertensive response to captopril [38].
Regarding the intracellular effect of ACE inhibitors, the drugs wereobserved to have an ACE independent effect [47], and partly assert their effect through NO [97]. Thus, the lower bioavailability of NO in persons of Africanancestry [10, 12, 36, 37, 72, 79, 81], might contribute to the low efficacy of ACE inhibitors. Asto the cause of low NO bioavailability, G6PD deficiency [79, 80], and low L-Arginine [69, 81], associated with enhanced creatine biosynthesis with highcreatine kinase [12, 72], have been suggested.
G6PD is the first and rate-limiting enzyme of the pentose phosphatepathway, thus serving as the principle source of cellular nicotinamideadenine dinucleotide phosphate-oxidase (NADPH), a cofactor for NOsynthase. Vascular endothelial cells constitutively express nitric oxidesynthase that forms NO in the presence of oxygen from the semi-essentialamino acid L-arginine. NO synthase binds NADPH, flavin adeninedinucleotide, flavin mononucleotide, L-arginine, a heme moiety andtetrahydrobiopterin. Tetrahydrobiopterin synthesis itself is alsodependent on available NADPH [80].
In line with this, G6PD deficiency, reported in up to 25% of persons ofAfrican ancestry [79], has been shown to reduce NO bioavailability invitro[80]. In addition, the high creatine synthesis associated with thehigh creatine kinase activity found in persons of African ancestry [11, 12, 72, 73], is thought to hamper the bioavailability of the precursorL-arginine shared with nitric oxide synthase (Figure 2). Thus, high CK has been shown to be associated with lowvascular NO bioavailability in vitro[72], and L-Arginine was found to be low in persons of Africanancestry [69], with supplementation restoring NO bioavailability invivo[81]. However, there are no clinical data yet that associate theresponse of ACE inhibitors to high CK or low NO.
Pharmacogenomic factors studied include polymorphisms in the ACEgene. The ACE insertion/deletion genotype ACE DD (30%of all participants; 33% of all participants of African ancestry,n = 13,070) had a poorer response to lisinopril treatmentthan to any of the other three drugs in the GenHAT study. However, theeffect was small, a difference of 0.85 mm Hg systolic (SE 0.51) and 0.50mm Hg diastolic (SE 0.28), with “similar” results reportedfor the subgroup analysis for patients of African ancestry [28].
In the AASK study, participants randomized to ramipril(n = 347) were genotyped at three polymorphisms onACE, downstream from the ACE insertion/deletionpolymorphism: G12269A, C17888T and G20037A. Only participants with ahomozygous genotype at G12269A and C17888T, and randomized to the usualmean arterial pressure goal (≤107 mm Hg) reached a blood pressuregoal significantly faster than those with a heterozygous genotype(adjusted hazard ratio respectively 1.86; 95% CI 1.32 to 3.23, and 1.49;95% CI 1.01 to 2.13, potentially due to linkage disequilibrium withACE I/D [23].
Finally, in a study including patients of African ancestry(n = 77), functional variants in the promoter region of theangiotensinogen gene (−217G = > A and −20A= > C) were assessed. Patients with the AA genotype ofthe −217G= > A variant treated with enalapril orlisinopril showed no significant decrease in blood pressure (systolicblood pressure + 0.84 (SD 2.89),P = 0.78; diastolic blood pressure −0.47 (SD1.74), P = 0.79); while patients with at least onecopy of the −217G allele developed respectively a 7.23 (1.55) and5.38 (1.12) mm Hg decrease (P <0.01). Similarly, in patientswith the −20AA genotype no change in blood pressure occurred,whereas in those patients with at least one copy of the −20Callele, systolic blood pressure decreased in response to ACE inhibitortherapy. In line with this, patients with at least one copy of both the−217G and the −20C allele developed substantial decreases inblood pressure (change in mean ambulatory blood pressure, mm Hg: SBP−14.08 +/− 3.72, P <0.01; DBP −9.62+/− 2.74, P <0.01) [26].
Other drugs affecting the RAAS system
Angiotensin receptor blockers are also less effective in patients of Africanancestry as compared to calcium blockers and diuretics [3]. In one study, the mean plasma concentration and eliminationhalf-life of irbesartan were 20 to 25% higher in persons of African than ofEuropean ancestry, while the peak plasma concentration was comparablebetween the two groups [68]. As with ACE inhibitors, ancestry was superior to renin profilingto predict the response to candesartan [22]. Finally, the aldosterone antagonist eplerenone was moreeffective than losartan in patients of African ancestry, and equallyeffective as in patients of European ancestry in one trial [84], despite similar or lower plasma aldosterone levels reported inpersons of African, compared to European ancestry [29, 84]. As stated above, renin levels did not predict the response tospironolactone (100 to 400 mg/d) [65].
β-adrenergic blockers
Clinical efficacy
The efficacy of systolic blood pressure lowering of β-adrenergicblockade as monotherapy in uncomplicated essential hypertension is notsignificantly different from placebo in patients of African ancestry,and some trials report significant placebo corrected increase in bloodpressure with β-adrenergic blockade in this population group [3, 92] The main side effects are metabolic, including higher glucoselevels [3].
Environmental factors
To our knowledge, there are no environmental factors reported that mayhelp explain the attenuated blood pressure lowering response of patientsof African ancestry to β-adrenergic blockade. In the TAIM study,adding sodium restriction (mean 100 mmol/day) to an atenolol regimen(usual/sodium restricted diet: atenolol n = 22/29; placebon = 26/19) did not enhance the blood pressure loweringeffect [70].
Pharmacokinetics
Studies on the differences in the pharmacokinetics of β-adrenergicblockers based on ancestry yielded heterogeneous results. Oral clearanceof L-propranolol was reported to be similar [56], or higher in persons of African, than in persons ofEuropean, ancestry (respectively 28 ml/min/kg, SD 8; vs 21, SD 7;P <0.05) [52], with similar, or up to 25% lower peak plasma concentrations [52, 56]. In line with this, hepatic metabolism of propranolol viaside chain oxidation, 4-hydroxylation or R-propranolol glucuronidationwas observed to be higher in persons of African than in those ofEuropean ancestry [44]. However, propranolol clearance after intravenous infusion(0.1 mg/kg), was similar in one study [61]. On the other hand, around 30% higher plasma concentrationswere found after 100 mg oral metoprolol in an indirect comparisonbetween subjects of African vs European ancestry, respectively 154 ng/mlvs 117 at t = 3 h [59], while others observed no significant differences in plasmapeak plasma concentrations or systemic clearance [55]. Also, metabolism of metoprolol via CYP2D6 assessed with anoral dose of 200 mg, given to men of African and European ancestry (10in each group) was not significantly different [45]. Finally, pharmacokinetic studies of pindolol yielded similarresults in both groups [64, 67].
Pharmacodynamics
The attenuated response of persons of African ancestry toβ-adrenergic blockers was extensively studied. As renin loweringcontributes to the antihypertensive effect of β-adrenergicblockers, these drugs were expected to be less effective in subjects ofAfrican ancestry [8]. Indeed, renin correlated with the blood pressure loweringresponse to atenolol 50 to 100 mg/d in a study including 67 subjects (33of African ancestry) [53]. However, renin did not predict the response to propranolol(80 to 640 mg/d) in 215 participants (132 of African ancestry) of aVeterans Administration study [15]. The relative contribution of renin vs African ancestry(β ± SE) was calculated in multivariableregression analysis, to be respectively−4.05 ± 0.84 vs−7.45 ± 1.53; both P <0.01 [15]. Finally, in a study of 335 subjects (152 of Africanancestry), therapeutic responses to atenolol 25 to 100 mg wereconsistent with a baseline renin profile, but age-ancestry subgroupprofiling was a better predictor of response [38].
β-blockers are thought to lower blood pressure predominantly througha reduction in cardiac contractility and heart rate. While early studiesfound a reduced sensitivity to isoprenaline in healthy men of Africanancestry [49], reports on changes in heart rate after β-blockers inhealthy volunteers were conflicting, with either a greater response inpersons of African ancestry (to oral propranolol 240 mg/d); [75] an attenuated response (to intravenous propranolol up to 0.15mg/kg [78], or metoprolol 50 μg/mL); [55] or no significant difference between groups (to intravenouspropranolol 0.15 mg/kg) [49]. We retrieved no studies in hypertensives.
Pharmacogenomic studies focussed on the frequency of occurrence of theresponsive β1-receptor (ADRB1) genotype Arg 389/Ser 49 inpersons of African ancestry, which was associated with greater bloodpressure lowering responses to β-adrenergic blockade in otherpopulation subgroups [14].
In one small study, including 40 subjects (10 of African ancestry)patients homozygous for Arg at codon 389 had a nearly three-fold greaterreduction in daytime diastolic blood pressure (−13.3% +/−8.4% versus −4.5% +/− 8.2%, P <0.01) comparedwith those who carried the variant allele, and Ser49-Arg389/Ser49-Arg389diplotype demonstrated a decline in blood pressure of 14.7 mm Hg versus0.5 mm Hg in patients with the Gly49-Arg389/Ser49-Gly389 diplotype, thiswas independent of ancestry [74].
In addition, Kurnik et al. studied sensitivity toβ-blockade by the attenuation of exercise-induced tachycardia in165 subjects (73 of African ancestry), and found that heart ratereduction was greatest in the Arg389/Arg389 group, intermediate in theheterozygotes, and smallest in the Gly389/Gly389 group; this effect wasseen in both ancestry groups. Carriers of the responsive Arg389/Ser49haplotype, had a 27% greater adjusted reduction in heart rate at maximalexercise (mean difference, 3.7 bpm; 95% CI, 1.2 to 6.2; P<0.01). However, differences in sensitivity to the β1-blockeratenolol persisted after accounting for different distributions offunctional genetic β1-receptor variants, suggesting that additionalfactors contribute to the differences found between ancestry groups [20].
The AASK study yielded conflicting results as time to reach the targetmean arterial pressure of 107 mm Hg with metoprolol (329 participantsrandomized) was not significantly different for Ser49 or Gly49 variants.In contrast with studies in other population subgroups, the“hazard” ratio of reaching goal blood pressure was lower,0.68 (95% CI 0.50 to 0.93) in individuals with at least one‘responsive’ Arg389 allele compared to individuals withGly389/Gly389 [14].
Finally, a series of pharmacogenomics studies did not further explain whypatients of African ancestry respond less to β-adrenergic blockade.The G-protein-coupled receptor kinase 5 (GRK5) codes for aserine/threonine kinase that phosphorylates and desensitizesG-protein-coupled receptors. However, in a study of 154 healthy subjects(69 of African ancestry), GRK5 Gln41Leu polymorphism, presentin approximately 40% of the persons of African and 2% of individualsEuropean of ancestry, did not affect the response to atenolol [18]. Furthermore, polymorphisms of the GRK2 gene(ADRBK1) and GRK5 Gln41Leu polymorphisms, studied in 418patients (167 of African ancestry) from the PEAR study did not affectthe blood pressure response to atenolol [77]. Finally, the polymorphisms Arg65Leu, Ala142Val, andAla486Val of the G protein-coupled receptor kinase gene, GRK4,were studied in the AASK Study [19]. Only in men randomized to the usual blood pressure goal(mean arterial pressure 102 to 107 mm Hg), the adjusted“hazard” ratio to reach goal blood pressure with metoprololwas 1.54 (95% CI 1.11 to 2.44; P <0.01) with Ala142Val.There was no association between GRK4 polymorphisms and bloodpressure response to metoprolol in women. Thus, despite extensiveresearch, there is no clear pharmacogenomic evidence why patients ofAfrican ancestry may have a differential response to β-adrenergicblockade.
An important aspect of β-blocker therapy is that inhibition ofβ2-mediated vasodilation by β-adrenergic blockers may induceperipheral vasoconstriction and blood pressure increase, thuscounteracting the antihypertensive effect [3, 92]. (Nebivolol, a β-adrenergic blocker that generatesintravascular NO is reported to have less vasoconstrictive effect) [16, 27]. β2-adrenergic effects were addressed in the followingstudies. A blunted forearm flow response was reported in subjects ofAfrican vs European ancestry after intra-arterial infusion ofisoprenaline, a nonselective β-adrenergic agonist (respectively10.9 (SE 1.7) with African, versus 14.9 (1.5) mL/min/dL with Europeanancestry; P <0.01 [37], with similar results in an independent study [42]. However, lymphocyte β-2-adrenergic receptor density wasfound similar in subjects of African compared to European ancestry(African, 19.2 +/− 2.2 fmol/mg protein; European, 15.2 +/−1.4 fmol/mg protein) [54], with a lower affinity of the β2-receptor forpropranolol in persons of African ancestry [51].
Studies on differences in intracellular cAMP production as part of theintracellular signaling cascade after receptor stimulation yieldedconflicting results. Lower, as well as higher, baseline andisoproterenol stimulated cAMP levels were found in subjects of Africancompared to European ancestry [54, 62], and men of African ancestry with the highest lymphocyteβ2-adrenergic agonists mediated cAMP production had the greatestblood pressure increases during antagonist (metoprolol) therapy [48].
The intracellular signaling pathway after β2-adrenergic stimulationand cAMP production eventually leads to inhibition of myosin light chainkinase activity and vasodilation [91]. β-blockers may thus promote peripheralvasoconstriction. Moreover, there is evidence of high vascular smoothmuscle creatine kinase in persons of African ancestry. The enzyme CKrapidly provides ATP for enzymes leading to vasoconstriction, includingmyosin light chain kinase [11, 12, 72, 73]. Hence, high activity of CK may facilitate pressor responseswith β-blockers (Figure 2), but as yetthere are no clinical data to substantiate this.
α-1-adrenergic antagonists
There were only minor pharmacokinetic differences between subjects of African(n = 6) and European ancestry (n = 6) in trimazosinpharmacokinetics, with the latter having a larger volume of distribution, and alonger terminal elimination half-life for the metabolite, 1-hydroxy-trimazosin [60]. Furthermore, profiling based on age and ancestry was shown to besuperior to renin levels in predicting the magnitude of the antihypertensiveresponse to prazosin [38].
Discussion
Why do hypertensive patients of African ancestry generally respond better todiuretics and calcium blockers and less well to ACE and β-adrenergic blockade?Many clinicians use the self-defined ancestry of a patient as a clinical guide toselect antihypertensive drugs [5], but considerable overlap in response is known to occur between ancestrygroups [3, 6–8]. Therefore, many health care workers and patients object to usingancestry as a proxy for drug response [7, 8], and it is advocated that reduction of blood pressure and relatedmortality should be achieved through individual treatment options [5, 7, 8]. However, to reach this end, ethno-cultural and biological differences indrug response behind the surrogate measures of ‘ancestry’ or‘ethnicity’ need to be identified.
To our knowledge, this is the first systematic review on environmental,pharmacokinetic and pharmacodynamic factors that may contribute to the differentialclinical response to different types of drugs observed in patients of Africanancestry. In this paper, we also addressed genetic variation thought to affectpharmacokinetic and pharmacodynamic mechanisms, of which phase 1 and phase 2 drugmetabolism and receptor function have been most extensively studied.
However, the magnitude of the effects of variation in single candidate genes onantihypertensive drug responses appears to be very modest, accounting for only asmall percentage of total variation in response when reported (<5%). Also, wefound considerable heterogeneity in the direction of the effect across sex andancestry groups. Studies of polymorphisms may reflect inheritance of a locus inlinkage disequilibrium with the gene variation. Because linkage disequilibrium isaffected by the population’s history, true associations due to linkagedisequilibrium may yield conflicting results in two separate populations [98]. No unique mutation was by itself predictive of the therapeutic responseto these drugs, and even the combined effects of polymorphisms did not account forenough variation in response to be clinically useful.
Differences in pharmacodynamics were most consistent, mainly related to thepathophysiology and clinical characteristic of hypertension in patients of Africanancestry. In this regard, new views have developed that expand the classicalpathophysiology of patients of African ancestry to have low renin hypertension [2, 8]. Low renin in itself does not explain the greater occurrence ofhypertension or the enhanced vascular contractility reported in this group [11], and in the presented data, profiling based on age and ancestry was equalor superior to renin in predicting drug responses. Recent data point to a centralrole for the balance between NO bioavailability and creatine kinase activity [10, 12, 16, 31, 72, 79–81]. The NO and CK systems share a common precursor in L-Arginine, anddisplay antagonizing effects with mutual inhibition (Figure 2). NO inhibits CK, lowers blood pressure and promotes cardiovascularhealth [11, 12, 81, 94]. High CK activity is thought to promote salt retention and vascularcontractility, with low renin as an epiphenomenon [11, 12, 72]. Cytoplasmic CK is tightly bound near ATPases, such asNa+K+ ATPase and myosin ATPase, to rapidly transfer aphosphoryl group from creatine phosphate to adenosine diphosphate (ADP) insitu, and generate ATP near these ATPases, thereby facilitating iontransport and muscle contractility [11, 12, 88–90]. The high creatine synthesis associated with high creatine kinaseactivity demands L-Arginine, which is thought to lower NO bioavailability [12, 72]. In line with this, CK is the main predictor of blood pressure in thegeneral population [11, 12, 99], and of failure of antihypertensive therapy [100]. Patients of African ancestry are reported to have low NO bioavailability [10], high CK activity [11, 12, 72, 73], and low L-arginine [69], with restored NO bioavailability upon L-Arginine supplementation [81]. However, although it is plausible that inter-individual differences inblood pressure lowering efficacy of drugs could be related to the balance between NOand CK activity, with lower efficacy of drugs that require NO synthesis (such as ACEinhibitors), or promote CK-dependent vasoconstriction (β-adrenergic blockers),and higher efficacy of drugs that counteract CK (diuretics and calcium blockers),there are no further clinical data yet to substantiate this. Hitherto, self-definedancestry remains the best predictor of responses to antihypertensive drugs, and isshown superior to renin status.
The main strength of this study is that this is the first systemic review, designedto assess potential causes for the different responses of patients of Africanancestry to antihypertensive drugs, including all published papers without languagerestriction, and considering salt intake, recent development in pathophysiology andpharmacogenomics, as well as resulting differences in pharmacokinetics andpharmacodynamics. Our systematic approach reduces over-interpretation of study data,and increases the transparency and reproducibility of the synthesis [13].
Using this rigid methodology, the data on potential predictors of blood pressureresponse in patients of African ancestry are far less conclusive than in previouslypublished, non-systematic overviews [6, 8, 98], with self-defined ancestry remaining the best predictor of responses toantihypertensive drugs. Although there is considerable heterogeneity among personsof sub-Saharan African descent, because of observed group differences in risk forhypertension, the field of hypertension continues to treat this group as a distinctbiological entity [101]. We included environmental as well as biological factors, but we areaware that in a real world setting, differences in access to care, clinicalmanagement and adherence to treatment may have more impact on morbidity andmortality of patients of African ancestry than the differential response toantihypertensive drugs [102]. Still, in our focus on the effect of drug therapy on blood pressure, weaddress the most practical aspect of treatment. Lowering blood pressure is the mostcost-effective way to reduce the morbidity and mortality of hypertension, andchoosing highly effective drugs early in the treatment procedure helps achieve earlyadequate blood pressure lowering and leads to greater adherence [5, 8]. We also note that for many patients, this would mean using initialcombination therapy [5], but there are insufficient data available to address differences inpharmacokinetic and pharmacodynamics of combination therapy based on ancestry.
Conclusions
Patients of African ancestry tend to suffer from more severe hypertension,characterized by enhanced vascular contractility and salt retaining capacity,therapy resistance, and higher morbidity and mortality of the condition and itscomplications. Because of the need for individual treatment options, as well as theincreasing objections to the use of ancestry as a surrogate marker for therapeuticresponses, we systematically gathered evidence on biomarkers that may predict theresponse of individual persons of African ancestry to different types ofantihypertensive drugs. However, pharmacogenomics yield heterogeneous, insufficientevidence, and the low renin levels found with greater frequency in patients ofAfrican ancestry do not, or do not adequately, predict responses to antihypertensivedrugs. Finally, there are no convincing clinical data yet of the emerging paradigmthat low NO bioavailability and associated high cellular ATP buffer capacity predictthe response to specific antihypertensive drugs. Currently, self-identifiedethno-geographic ancestry remains the best available predictor of blood pressurelowering responses to antihypertensive drugs.
Abbreviations
- AASK:
-
The African-American study of kidney disease and hypertension
- ACE or gene ACE:
-
Angiotensin converting enzyme
- ACE-i:
-
ACE inhibitor
- ADD1:
-
Alpha-adducin gene
- ADP:
-
adenosine diphosphate
- ADRB1:
-
β-1-adrenergic receptor gene
- AGT:
-
Angiotensinogen gene
- AGTR1 or AT1R:
-
Angiotensin II receptor type I gene
- AIM:
-
African index medicus
- ALLHAT:
-
The antihypertensive and lipid-lowering treatment toprevent heart attack trial
- BP:
-
Blood pressure
- Ca-blockers or CaB:
-
Calcium channelblocker
- cAMP:
-
Adenosine cyclic 3′,5′-(monophosphate)
- cGMP:
-
Guanosinecyclic 3′,5′-(monophosphate)
- CK:
-
Creatine kinase
- Creatine ~ P:
-
Creatine phosphate
- CYP11B2:
-
Aldosterone synthase gene
- EMA:
-
European Medicines Agency
- FDA:
-
The Food and Drug Administration
- GenHAT:
-
Thegenetics of hypertension-associated treatment study
- GNB3 and GNAS1:
-
G-proteinsubunits genes
- HCT:
-
Hydrochlorothiazide
- INVEST-GENES:
-
The InternationalVerapamil/trandolapril study (INVEST) genetic substudy
- LC:
-
Light chain
- LILACS:
-
Literatura Latino-Americana y del Caribe en Ciencias de la Salud
- MAP:
-
Mean arterialblood pressure
- MLCP:
-
Myosin light chain phosphatase
- NADPH:
-
Nicotinamide adeninedinucleotide phosphate-oxidase
- NO:
-
Nitric oxide
- NOS3:
-
Endothelial nitric oxidesynthase gene
- PEAR:
-
The pharmacogenomic evaluation of antihypertensive responses
- RAAS:
-
Renin-ngiotensin-aldosterone system
- REN:
-
Renin gene
- SER:
-
Sarcoendoplasmic reticulum
- TAIM:
-
The trial of antihypertensive interventions andmanagement.
References
Ford ES: Trends in mortality from all causes and cardiovascular disease amonghypertensive and nonhypertensive adults in the United States. Circulation. 2011, 123: 1737-1744. 10.1161/CIRCULATIONAHA.110.005645.
Opie LH, Seedat YK: Hypertension in sub-Saharan African populations. Circulation. 2005, 112: 3562-3568. 10.1161/CIRCULATIONAHA.105.539569.
Brewster LM, van Montfrans GA, Kleijnen J: Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004, 141: 614-627. 10.7326/0003-4819-141-8-200410190-00009.
Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K: Higher cardiovascular disease prevalence and mortality among younger blackscompared to whites. Am J Med. 2010, 123: 811-818. 10.1016/j.amjmed.2010.04.020.
Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA: Management of high blood pressure in Blacks: an update of the InternationalSociety on Hypertension in Blacks consensus statement. Hypertension. 2010, 56: 780-800. 10.1161/HYPERTENSIONAHA.110.152892.
Johnson JA: Ethnic differences in cardiovascular drug response: potential contribution ofpharmacogenetics. Circulation. 2008, 118: 1383-1393. 10.1161/CIRCULATIONAHA.107.704023.
Sehgal AR: Overlap between whites and blacks in response to antihypertensive drugs. Hypertension. 2004, 43: 566-572. 10.1161/01.HYP.0000118019.28487.9c.
Jamerson KA: Rationale for angiotensin II receptor blockers in patients with low-reninhypertension. Am J Kidney Dis. 2000, 36: S24-S30.
Schwartz GL, Turner ST: Pharmacogenetics of antihypertensive drug responses. Am J Pharmacogenomics. 2004, 4: 151-160. 10.2165/00129785-200404030-00002.
Kalinowski L, Dobrucki IT, Malinski T: Race-specific differences in endothelial function: predisposition of AfricanAmericans to vascular diseases. Circulation. 2004, 109: 2511-2517. 10.1161/01.CIR.0000129087.81352.7A.
Brewster LM, Clark JF, van Montfrans GA: Is greater tissue activity of creatine kinase the genetic factor increasinghypertension risk in black people of sub-Saharan African descent?. J Hypertens. 2000, 18: 1537-1544. 10.1097/00004872-200018110-00002.
Brewster LM, Mairuhu G, Bindraban NR, Koopmans RP, Clark JF, van Montfrans GA: Creatine kinase activity is associated with blood pressure. Circulation. 2006, 114: 2034-2039. 10.1161/CIRCULATIONAHA.105.584490.
Rodgers M, Arai L, Popay J, Britten N, Roberts H, Petticrew M, Sowden A: Testing methodological guidance on the conduct of narrative synthesis insystematic reviews: effectiveness of interventions to promote smoke alarmownership and function. Evaluation. 2009, 15: 49-73. 10.1177/1356389008097871.
Lee J, Aziz H, Liu L, Lipkowitz M, O’Connor DT, Richard E, Brophy V, Wassel CL, Blantz R, Bhatnagar V: β(1)-adrenergic receptor polymorphisms and response to β-blockadein the African-American study of kidney disease and hypertension (AASK). Am J Hypertens. 2011, 24: 694-700. 10.1038/ajh.2011.39.
Turner ST, Schwartz GL, Chapman AB, Beitelshees AL, Gums JG, Cooper-DeHoff RM, Boerwinkle E, Johnson JA, Bailey KR: Plasma renin activity predicts blood pressure responses to beta-blocker andthiazide diuretic as monotherapy and add-on therapy for hypertension. Am J Hypertens. 2010, 23: 1014-1022. 10.1038/ajh.2010.98.
Merchant N, Searles CD, Pandian A, Rahman ST, Ferdinand KC, Umpierrez GE, Khan BV: Nebivolol in high-risk, obese African Americans with stage 1 hypertension:effects on blood pressure, vascular compliance, and endothelial function. J Clin Hypertens. 2009, 11: 720-725. 10.1111/j.1751-7176.2009.00198.x.
Bhatnagar V, Garcia EP, O’Connor DT, Brophy VH, Alcaraz J, Richard E, Bakris GL, Middleton JP, Norris KC, Wright J, Hiremath L, Contreras G, Appel LJ, Lipkowitz MS: CYP3A4 and CYP3A5 polymorphisms and blood pressure response to amlodipineamong African-American men and women with early hypertensive renaldisease. Am J Nephrol. 2010, 31: 95-103. 10.1159/000258688.
Kurnik D, Cunningham AJ, Sofowora GG, Kohli U, Li C, Friedman EA, Muszkat M, Menon UB, Wood AJ, Stein CM: GRK5 Gln41Leu polymorphism is not associated with sensitivity tobeta(1)-adrenergic blockade in humans. Pharmacogenomics. 2009, 10: 1581-1587. 10.2217/pgs.09.92.
Bhatnagar V, O’Connor DT, Brophy VH, Schork NJ, Richard E, Salem RM, Nievergelt CM, Bakris GL, Middleton JP, Norris KC, Wright J, Hiremath L, Contreras G, Appel LJ, Lipkowitz MS: G-protein-coupled receptor kinase 4 polymorphisms and blood pressure responseto metoprolol among African Americans: sex-specificity and interactions. Am J Hypertens. 2009, 22: 332-338. 10.1038/ajh.2008.341.
Kurnik D, Li C, Sofowora GG, Friedman EA, Muszkat M, Xie HG, Harris PA, Williams SM, Nair UB, Wood AJ, Stein CM: Beta-1-adrenoceptor genetic variants and ethnicity independently affectresponse to beta-blockade. Pharmacogenet Genomics. 2008, 18: 895-902. 10.1097/FPC.0b013e328309733f.
Turner ST, Bailey KR, Fridley BL, Chapman AB, Schwartz GL, Chai HS, Sicotte H, Kocher JP, Rodin AS, Boerwinkle E: Genomic association analysis suggests chromosome 12 locus influencingantihypertensive response to thiazide diuretic. Hypertension. 2008, 52: 359-365. 10.1161/HYPERTENSIONAHA.107.104273.
Canzanello VJ, Baranco-Pryor E, Rahbari-Oskoui F, Schwartz GL, Boerwinkle E, Turner ST, Chapman AB: Predictors of blood pressure response to the angiotensin receptor blockercandesartan in essential hypertension. Am J Hypertens. 2008, 21: 61-66. 10.1038/ajh.2007.24.
Bhatnagar V, O’Connor DT, Schork NJ, Salem RM, Nievergelt CM, Rana BK, Smith DW, Bakris GL, Middleton JP, Norris KC, Wright JT, Cheek D, Hiremath L, Contreras G, Appel LJ, Lipkowitz MS: Angiotensin-converting enzyme gene polymorphism predicts the time-course ofblood pressure response to angiotensin converting enzyme inhibition in theAASK trial. J Hypertens. 2007, 25: 2082-2092. 10.1097/HJH.0b013e3282b9720e.
Langaee TY, Gong Y, Yarandi HN, Katz DA, Cooper-DeHoff RM, Pepine CJ, Johnson JA: Association of CYP3A5 polymorphisms with hypertension and antihypertensiveresponse to verapamil. Clin Pharmacol Ther. 2007, 81: 386-391. 10.1038/sj.clpt.6100090.
Davis BR, Arnett DK, Boerwinkle E, Ford CE, Leiendecker-Foster C, Miller MB, Black H, Eckfeldt JH: Antihypertensive therapy, the alpha-adducin polymorphism, and cardiovasculardisease in high-risk hypertensive persons: the Genetics ofHypertension-Associated Treatment Study. Pharmacogenomics J. 2007, 7: 112-122. 10.1038/sj.tpj.6500395.
Woodiwiss AJ, Nkeh B, Samani NJ, Badenhorst D, Maseko M, Tiago AD, Candy GP, Libhaber E, Sareli P, Brooksbank R, Norton GR: Functional variants of the angiotensinogen gene determine antihypertensiveresponses to angiotensin-converting enzyme inhibitors in subjects of Africanorigin. J Hypertens. 2006, 24: 1057-1064. 10.1097/01.hjh.0000226195.59428.57.
Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T: Nebivolol reduces nitroxidative stress and restores nitric oxidebioavailability in endothelium of black Americans. Circulation. 2005, 112: 3795-3801. 10.1161/CIRCULATIONAHA.105.556233.
Arnett DK, Davis BR, Ford CE, Boerwinkle E, Leiendecker-Foster C, Miller MB, Black H, Eckfeldt JH: Pharmacogenetic association of the angiotensin-converting enzymeinsertion/deletion polymorphism on blood pressure and cardiovascular risk inrelation to antihypertensive treatment: the Genetics ofHypertension-Associated Treatment (GenHAT) study. Circulation. 2005, 111: 3374-3383. 10.1161/CIRCULATIONAHA.104.504639.
Grim CE, Cowley AW, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, Krishnaswami S, Pausova Z, Roman R, Tremblay J, Kotchen TA: Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005, 45: 766-772. 10.1161/01.HYP.0000154364.00763.d5.
Frazier L, Turner ST, Schwartz GL, Chapman AB, Boerwinkle E: Multilocus effects of the renin-angiotensin-aldosterone system genes on bloodpressure response to a thiazide diuretic. Pharmacogenomics J. 2004, 4: 17-23. 10.1038/sj.tpj.6500215.
Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gokce N, Loscalzo J, Vita JA: Effects of black race on forearm resistance vessel function. Hypertension. 2002, 40: 195-201. 10.1161/01.HYP.0000024571.69634.ED.
Turner ST, Schwartz GL, Chapman AB, Boerwinkle E: C825T polymorphism of the G protein beta(3)-subunit and antihypertensiveresponse to a thiazide diuretic. Hypertension. 2001, 37: 739-743. 10.1161/01.HYP.37.2.739.
Ripley E, King K, Sica DA: Racial differences in response to acute dosing with hydrochlorothiazide. Am J Hypertens. 2000, 13: 157-164. 10.1016/S0895-7061(99)00168-5.
Damasceno A, Santos A, Pestana M, Serrão P, Caupers P, Soares-da-Silva P, Polónia J: Acute hypotensive, natriuretic, and hormonal effects of nifedipine insalt-sensitive and salt-resistant black normotensive and hypertensivesubjects. J Cardiovasc Pharmacol. 1999, 34: 346-353. 10.1097/00005344-199909000-00005.
He J, Klag MJ, Appel LJ, Charleston J, Whelton PK: The renin-angiotensin system and blood pressure: differences between blacksand whites. Am J Hypertens. 1999, 12: 555-562. 10.1016/S0895-7061(99)00030-8.
Jones DS, Andrawis NS, Abernethy DR: Impaired endothelial-dependent forearm vascular relaxation in blackAmericans. Clin Pharmacol Ther. 1999, 65: 408-412. 10.1016/S0009-9236(99)70135-9.
Cardillo C, Kilcoyne CM, Cannon RO, Panza JA: Attenuation of cyclic nucleotide-mediated smooth muscle relaxation in blacksas a cause of racial differences in vasodilator function. Circulation. 1999, 99: 90-95. 10.1161/01.CIR.99.1.90.
Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, Anderson RJ: Age-race subgroup compared with renin profile as predictors of blood pressureresponse to antihypertensive therapy. JAMA. 1998, 280: 1168-1172. 10.1001/jama.280.13.1168.
Cardillo C, Kilcoyne CM, Cannon RO, Panza JA: Racial differences in nitric oxide-mediated vasodilator response to mentalstress in the forearm circulation. Hypertension. 1998, 31: 1235-1239. 10.1161/01.HYP.31.6.1235.
Weir MR, Saunders E: Renin status does not predict the anti-hypertensive response toangiotensin-converting enzyme inhibition in African-Americans. J Hum Hypertens. 1998, 12: 189-194. 10.1038/sj.jhh.1000578.
Weir MR, Chrysant SG, McCarron DA, Canossa-Terris M, Cohen JD, Gunter PA, Lewin AJ, Mennella RF, Kirkegaard LW, Hamilton JH, Weinberger MH, Weder AB: Influence of race and dietary salt on the antihypertensive efficacy of anangiotensin-converting enzyme inhibitor or a calcium channel antagonist insalt-sensitive hypertensives. Hypertension. 1998, 31: 1088-1096. 10.1161/01.HYP.31.5.1088.
Stein CM, Lang CC, Nelson R, Brown M, Wood AJ: Vasodilation in black Americans: attenuated nitric oxide-mediatedresponses. Clin Pharmacol Ther. 1997, 62: 436-443. 10.1016/S0009-9236(97)90122-3.
Weir MR, Hall PS, Behrens MT, Flack JM: Salt and blood pressure responses to calcium antagonism in hypertensivepatients. Hypertension. 1997, 30: 422-427. 10.1161/01.HYP.30.3.422.
Sowinski KM, Lima JJ, Burlew BS, Massie JD, Johnson JA: Racial differences in propranolol enantiomer kinetics following simultaneousi.v. and oral administration. Br J Clin Pharmacol. 1996, 42: 339-346.
Johnson JA, Burlew BS: Metoprolol metabolism via cytochrome P4502D6 in ethnic populations. Drug Metab Dispos. 1996, 24: 350-355.
Sowunmi A, Rashid TJ, Akinyinka OO, Renwick AG: Ethnic differences in nifedipine kinetics: comparisons between Nigerians,Caucasians and South Asians. Br J Clin Pharmacol. 1995, 40: 489-493.
Weir MR, Gray JM, Paster R, Saunders E: Differing mechanisms of action of angiotensin-converting enzyme inhibition inblack and white hypertensive patients. Hypertension. 1995, 26: 124-130. 10.1161/01.HYP.26.1.124.
Johnson JA, Akers WS, Miller ST, Applegate WB: Lymphocyte beta 2-receptor activity, metoprolol kinetics, and response tometoprolol in hypertensive black men. Pharmacotherapy. 1995, 15: 150-157.
Johnson JA, Burlew BS, Stiles RN: Racial differences in beta-adrenoceptor-mediated responsiveness. J Cardiovasc Pharmacol. 1995, 25: 90-96. 10.1097/00005344-199501000-00015.
Cappuccio FP, Markandu ND, Singer DR, MacGregor GA: Amlodipine and lisinopril in combination for the treatment of essentialhypertension: efficacy and predictors of response. J Hypertens. 1993, 11: 839-847. 10.1097/00004872-199308000-00011.
Johnson JA: Racial differences in lymphocyte beta-receptor sensitivity to propranolol. Life Sci. 1993, 53: 297-304. 10.1016/0024-3205(93)90748-R.
Johnson JA, Burlew BS: Racial differences in propranolol pharmacokinetics. Clin Pharmacol Ther. 1992, 51: 495-500. 10.1038/clpt.1992.53.
Wright JT, DiPette DJ, Goodman RP, Townsend R, McKenney JM: Renin profile, race, and antihypertensive efficacy with atenolol andlabetalol. J Hum Hypertens. 1991, 5: 193-198.
Stein M, O’Malley K, Kilfeather S: Ethnic differences in cyclic AMP accumulation: effect on alpha 2, beta 2, andprostanoid receptor responses. Clin Pharmacol Ther. 1990, 47: 360-365. 10.1038/clpt.1990.40.
Rutledge DR, Steinberg J, Cardozo L: Racial differences in drug response: isoproterenol effects on heart ratefollowing intravenous metoprolol. Clin Pharmacol Ther. 1989, 45: 380-386. 10.1038/clpt.1989.44.
Sharoky M, Perkal M, Turner R, Lesko LJ: Steady state relative bioavailability and pharmacokinetics of oralpropranolol in black and white North Americans. Biopharm Drug Dispos. 1988, 9: 447-456. 10.1002/bod.2510090503.
Hawkins DW, Dieckmann MR, Horner RD: Diuretics and hypertension in black adults. Arch Intern Med. 1988, 148: 803-805. 10.1001/archinte.1988.00380040043009.
Lettieri JT, Krol GJ, Yeh SC, Burkholder DE, Zinny M, O’Donnell D: The effects of age and race on nitrendipine pharmacokinetics andpharmacodynamics. J Cardiovasc Pharmacol. 1988, 12: S129-S132.
Iyun AO, Lennard MS, Tucker GT, Woods HF: Metoprolol and debrisoquin metabolism in Nigerians: lack of evidence forpolymorphic oxidation. Clin Pharmacol Ther. 1986, 40: 387-394. 10.1038/clpt.1986.195.
Vincent J, Elliott HL, Meredith PA, Reid JL: Racial differences in drug responses–a comparative study of trimazosinand alpha 1-adrenoceptor responses in normotensive Caucasians and WestAfricans. Br J Clin Pharmacol. 1986, 21: 401-408. 10.1111/j.1365-2125.1986.tb05214.x.
Venter CP, Joubert PH, Strydom WJ: Comparative pharmacokinetics of intravenous propranolol in black and whitevolunteers. J Cardiovasc Pharmacol. 1985, 7: 409-410. 10.1097/00005344-198503000-00029.
Venter CP, Daya S, Joubert PH, Strydom WJ: Ethnic differences in human lymphocytic cyclic AMP production afterisoprenaline stimulation and propranolol blockade. Br J Clin Pharmacol. 1985, 19: 187-190. 10.1111/j.1365-2125.1985.tb02630.x.
Freis ED, Materson BJ, Flamenbaum V: Comparison of propranolol or hydrochlorothiazide alone for treatment ofhypertension. III. Evaluation of the renin-angiotensin system. Am J Med. 1983, 74: 1029-1041. 10.1016/0002-9343(83)90812-4.
Juma FD: Pharmacokinetics of pindolol in Kenyan Africans. Eur J Clin Pharmacol. 1983, 25: 425-426. 10.1007/BF01037959.
Holland OB, Gomez-Sanchez C, Fairchild C, Kaplan NM: Role of renin classification for diuretic treatment of black hypertensivepatients. Arch Intern Med. 1979, 139: 1365-1370. 10.1001/archinte.1979.03630490029011.
Holland OB, Gomez-Sanchez CE, Kuhnert LV, Poindexter C, Pak CY: Antihypertensive comparison of furosemide with hydrochlorothiazide for blackpatients. Arch Intern Med. 1979, 139: 1015-1021. 10.1001/archinte.1979.03630460047016.
Salako LA, Falase AO, Ragon A, Adio RA: beta-Adrenoceptor blocking effects and pharmacokinetics of pindolol. A studyin hypertensive Africans. Eur J Clin Pharmacol. 1979, 15: 299-304. 10.1007/BF00558431.
European Medicines Agency: Scientific discussion. Aprovel/Karvea (Irbesartan). [http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500025747]
Glyn MC, Anderssohn M, Lüneburg N, Van Rooyen JM, Schutte R, Huisman HW, Fourie CM, Smith W, Malan L, Malan NT, Mels CM, Böger RH, Schutte AE: Ethnicity-specific differences in L-arginine status in South African men. J Hum Hypertens. 2012, 26: 737-743. 10.1038/jhh.2011.103.
Wassertheil-Smoller S, Davis BR, Oberman A, Blaufox MD, Langford H, Wylie-Rosett J, Hawkins M, Zimbaldi N: The TAIM study: sex-race differences in effects of diet and drugs oncardiovascular risk. Cardiovasc Risk Factors. 1991, 1: 427-435.
Chun TY, Bankir L, Eckert GJ, Bichet DG, Saha C, Zaidi SA, Wagner MA, Pratt JH: Ethnic differences in renal responses to furosemide. Hypertension. 2008, 52: 241-248. 10.1161/HYPERTENSIONAHA.108.109801.
Brewster LM, Taherzadeh Z, Volger S, Clark JF, Rolf T, Wolf H, Vanbavel E, van Montfrans GA: Ethnic differences in resistance artery contractility of normotensivepregnant women. Am J Physiol Heart Circ Physiol. 2010, 299: H431-H436. 10.1152/ajpheart.00919.2009.
Brewster LM, Coronel CM, Sluiter W, Clark JF, van Montfrans GA: Ethnic differences in tissue creatine kinase activity: an observationalstudy. PLoS One. 2012, 7: e32471-10.1371/journal.pone.0032471.
Johnson JA, Zineh I, Puckett BJ, McGorray SP, Yarandi HN, Pauly DF: Beta 1-adrenergic receptor polymorphisms and antihypertensive response tometoprolol. Clin Pharmacol Ther. 2003, 74: 44-52. 10.1016/S0009-9236(03)00068-7.
Sowinski KM, Burlew BS, Johnson JA: Racial differences in sensitivity to the negative chronotropic effects ofpropranolol in healthy men. Clin Pharmacol Ther. 1995, 57: 678-683. 10.1016/0009-9236(95)90231-7.
Duarte JD, Lobmeyer MT, Wang Z, Chapman AB, Gums JG, Langaee TY, Boerwinkle E, Turner ST, Johnson JA: Lack of association between polymorphisms in STK39, a putative thiazideresponse gene, and blood pressure response to hydrochlorothiazide. Pharmacogenet Genomics. 2010, 20: 516-519. 10.1097/FPC.0b013e32833b5958.
Lobmeyer MT, Wang L, Zineh I, Turner ST, Gums JG, Chapman AB, Cooper-DeHoff RM, Beitelshees AL, Bailey KR, Boerwinkle E, Pepine CJ, Johnson JA: Polymorphisms in genes coding for GRK2 and GRK5 and response differences inantihypertensive-treated patients. Pharmacogenet Genomics. 2011, 21: 42-49. 10.1097/FPC.0b013e328341e911.
Venter CP, Joubert PH: Ethnic differences in response to betaradrenoceptor blockade bypropranolol. J Cardiovasc Pharmacol. 1984, 6: 361-364. 10.1097/00005344-198403000-00024.
Gaskin RS, Estwick D, Peddi R: G6PD deficiency: its role in the high prevalence of hypertension and diabetesmellitus. Ethn Dis. 2001, 11: 749-754.
Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo J: Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidantstress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001, 15: 1771-1773.
Houghton JL, Philbin EF, Strogatz DS, Torosoff MT, Fein SA, Kuhner PA, Smith VE, Carr AA: The presence of African American race predicts improvement in coronaryendothelial function after supplementary L-arginine. J Am Coll Cardiol. 2002, 39: 1314-1322. 10.1016/S0735-1097(02)01781-3.
Pratt JH, Rebhun JF, Zhou L, Ambrosius WT, Newman SA, Gomez-Sanchez CE, Mayes DF: Levels of mineralocorticoids in whites and blacks. Hypertension. 1999, 34: 315-319. 10.1161/01.HYP.34.2.315.
Duarte JD, Turner ST, Tran B, Chapman AB, Bailey KR, Gong Y, Gums JG, Langaee TY, Beitelshees AL, Cooper-Dehoff RM, Boerwinkle E, Johnson JA: Association of chromosome 12 locus with antihypertensive response tohydrochlorothiazide may involve differential YEATS4 expression. Pharmacogenomics J. 2012, . [Epub ahead of print].
Flack JM, Oparil S, Pratt JH, Roniker B, Garthwaite S, Kleiman JH, Yang Y, Krause SL, Workman D, Saunders E: Efficacy and tolerability of eplerenone and losartan in hypertensive blackand white patients. J Am Coll Cardiol. 2003, 41: 1148-1155. 10.1016/S0735-1097(03)00054-8.
Rebbeck TR, Sankar P: Ethnicity, ancestry, and race in molecular epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2005, 14: 2467-2471. 10.1158/1055-9965.EPI-05-0649.
Greger R: Physiology of renal sodium transport. Am J Med Sci. 2000, 319: 51-62. 10.1097/00000441-200001000-00005.
Guerrero ML, Beron J, Spindler B, Groscurth P, Wallimann T, Verrey F: Metabolic support of Na + pump in apically permeabilized A6kidney cell epithelia: role of creatine kinase. Am J Physiol. 1997, 272: C697-C706.
Dzeja PP, Terzic A: Phosphotransfer networks and cellular energetics. J Exp Biol. 2003, 206: 2039-2047. 10.1242/jeb.00426.
Clark JF: The creatine kinase system in smooth muscle. Mol Cell Biochem. 1994, 133–134: 221-232.
Hardin CD, Raeymaekers L, Paul RJ: Comparison of endogenous and exogenous sources of ATP in fueling Ca2+ uptakein smooth muscle plasma membrane vesicles. J Gen Physiol. 1992, 99: 21-40. 10.1085/jgp.99.1.21.
Raina H, Zacharia J, Li M, Wier WG: Activation by Ca2+/calmodulin of an exogenous myosin light chain kinase inmouse arteries. J Physiol. 2009, 587: 2599-2612. 10.1113/jphysiol.2008.165258.
Veterans Administration Cooperative Study Group on AntihypertensiveAgents: Comparison of propranolol and hydrochlorothiazide for the initial treatmentof hypertension. I. Results of short-term titration with emphasis on racialdifferences in response. JAMA. 1982, 248: 1996-2003.
Stepien O, Zhang Y, Zhu D, Marche P: Dual mechanism of action of amlodipine in human vascular smooth musclecells. J Hypertens. 2002, 20: 95-102. 10.1097/00004872-200201000-00014.
Wu G, Morris SM: Arginine metabolism: nitric oxide and beyond. Biochem J. 1998, 336: 1-17.
Lee DL, Webb RC, Jin L: Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlightsfrom the recent literature. Hypertension. 2004, 44: 796-799. 10.1161/01.HYP.0000148303.98066.ab.
Wedler B, Brier ME, Wiersbitzky M, Gruska S, Wolf E, Kallwellis R, Aronoff GR, Luft FC: Sodium kinetics in salt-sensitive and salt-resistant normotensive andhypertensive subjects. J Hypertens. 1992, 10: 663-669.
Desta B, Vanhoutte PM, Boulanger CM: Inhibition of the angiotensin converting enzyme by perindoprilat and releaseof nitric oxide. Am J Hypertens. 1995, 8: 1S-6S. 10.1016/0895-7061(95)00026-L.
Turner ST, Schwartz GL, Boerwinkle E: Personalized medicine for high blood pressure. Hypertension. 2007, 50: 1-5. 10.1161/HYPERTENSIONAHA.107.087049.
Johnsen SH, Lilleng H, Wilsgaard T, Bekkelund SI: Creatine kinase activity and blood pressure in a normal population: theTromsø study. J Hypertens. 2011, 29: 36-42. 10.1097/HJH.0b013e32834068e0.
Oudman I, Kewalbansingh P, van Valkengoed I, Zwinderman AH, Clark JF, van Montfrans GA, Brewster LM: Creatine kinase is associated with failure of hypertension treatment. J Hypertens. 2013, 31: 1025-1031. 10.1097/HJH.0b013e32835f5c29.
Myers HF, McClure H: Psychosocial factors in hypertension in blacks: the case for an interactionalperspective. Pathophysiology of Hypertension in Blacks. Edited by: Fray JCS, Douglas JG. 1993, Oxford: Oxford University Press, 90-106.
MacMahon S, Alderman MH, Lindholm LH, Liu L, Sanchez RA, Seedat YK: Blood-pressure-related disease is a global health priority. Lancet. 2008, 371: 1480-1482. 10.1016/S0140-6736(08)60632-7.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1741-7015/11/141/prepub
Acknowledgments
LMB is a recipient of a VENI fellowship (grant number 916.10.156) awarded by thenational Netherlands Organisation for Scientific Research (NWO) as part of itsInnovational Research Incentives Scheme. The funding body had no role in thestudy design, or in the collection, analysis, and interpretation of data, thewriting of the manuscript; or in the decision to submit the manuscript forpublication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
YKS declare to have no competing interests. LMB is an inventor on NL patentWO/2012/138226 (filed).
Authors’ contributions
Both authors have made substantial contributions to conception and design. LMBperformed the search. Both authors contributed to extraction, analysis andinterpretation of the data, and to drafting the manuscript. The authors read andapproved the final manuscript and have given final approval of the version to bepublished.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative CommonsAttribution License (http://creativecommons.org/licenses/by/2.0), whichpermits unrestricted use, distribution, and reproduction in any medium, provided theoriginal work is properly cited.
About this article
Cite this article
Brewster, L.M., Seedat, Y.K. Why do hypertensive patients of African ancestry respond better to calciumblockers and diuretics than to ACE inhibitors and β-adrenergic blockers? Asystematic review. BMC Med 11, 141 (2013). https://doi.org/10.1186/1741-7015-11-141
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1741-7015-11-141