Abstract
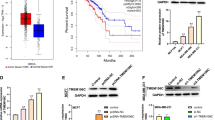
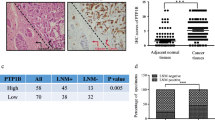
Breast cancer already taken the first place of incidence in Chinese female cancer patients. TRPM8 is found to be over-expressed in breast cancer, but whether it promotes breast cancer aggressiveness remains unknown. In our study, TRPM8 was identified highly expressing in all the tested breast cancer cell lines including MCF-7, T47D, MDA-MB-231, BT549, SKBR3 and ZR-75-30, while it just could be detected in MCF-10A, the normal breast epithelial cell. Then four pairs of clinical samples were analyzed using Western blotting and the result showed that TRPM8 expression is higher in tumor tissues than in adjacent nontumor tissues. Subsequently, we established TRPM8 high-expressing MCF-7 cell line and TRPM8 knockout MDA-MB-231 cell line to explore expression status of cancer-related proteins. The Western blotting and immunofluorescence analysis outcomes demonstrated that TRPM8 might influence cancer cell metastasis by regulating the EMT phenotype via activating AKT/GSK-3β pathway, and the hypothesis had been supported by cell function tests. All the results demonstrated that TRPM8 significantly up-expressed in breast cancer cells and promoted their metastasis by regulating EMT via activating AKT/GSK-3β pathway, indicating TRPM8 gets the prospects of to be developed as medication or diagnostic indicator to be applied in clinical work.




Similar content being viewed by others
References
Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–34. doi:10.1016/S0140-6736(13)60140-3.
Engebraaten O, Vollan HK, Borresen-Dale AL. Triple-negative breast cancer and the need for new therapeutic targets. Am J Pathol. 2013. doi:10.1016/j.ajpath.2013.05.033.
Lee GS, Jeung EB. Uterine TRPV6 expression during the estrous cycle and pregnancy in a mouse model. Am J Physiol Endocrinol Metab. 2007;293(1):E132–8. doi:10.1152/ajpendo.00666.2006.
Bolanz KA, Hediger MA, Landowski CP. The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther. 2008;7(2):271–9. doi:10.1158/1535-7163.MCT-07-0478.
Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772(8):805–12. doi:10.1016/j.bbadis.2007.02.002.
Gkika D, Prevarskaya N. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochim Biophys Acta. 2009;1793(6):953–8. doi:10.1016/j.bbamcr.2008.11.010.
Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772(8):937–46. doi:10.1016/j.bbadis.2007.05.006.
McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–8. doi:10.1038/nature719.
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–15.
Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152(7):1459–68. doi:10.1016/j.pain.2011.01.027.
Brignell JL, Chapman V, Kendall DA. Comparison of icilin- and cold-evoked responses of spinal neurones, and their modulation of mechanical activity, in a model of neuropathic pain. Brain Res. 2008;1215:87–96. doi:10.1016/j.brainres.2008.03.072.
Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol CB. 2006;16(16):1591–605. doi:10.1016/j.cub.2006.07.061.
Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6(9):e25894. doi:10.1371/journal.pone.0025894.
Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61(9):3760–9.
Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, et al. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer. 2010;10:212. doi:10.1186/1471-2407-10-212.
Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP calcium channel and breast cancer: expression, role and correlation with clinical parameters. Bull Cancer. 2012;99(6):655–64. doi:10.1684/bdc.2012.1595.
Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2011;28(5):813–22. doi:10.1159/000335795.
Malkia A, Morenilla-Palao C, Viana F. The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface. Curr Pharm Biotechnol. 2011;12(1):54–67.
Voulgari A, Pintzas A. Epithelial–mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796(2):75–90. doi:10.1016/j.bbcan.2009.03.002.
Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial–mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15(2):169–90. doi:10.1007/s10911-010-9181-1.
Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial–mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):201–12. doi:10.1007/s10911-010-9177-x.
Jin H, Morohashi S, Sato F, Kudo Y, Akasaka H, Tsutsumi S, et al. Vimentin expression of esophageal squamous cell carcinoma and its aggressive potential for lymph node metastasis. Biomed Res. 2010;31(2):105–12.
Cai Z, Wang Q, Zhou Y, Zheng L, Chiu JF, He QY. Epidermal growth factor-induced epithelial–mesenchymal transition in human esophageal carcinoma cells—a model for the study of metastasis. Cancer Lett. 2010;296(1):88–95. doi:10.1016/j.canlet.2010.03.020.
Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–54. doi:10.1038/sj.onc.1209091.
Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275(47):36803–10. doi:10.1074/jbc.M005912200.
Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63(9):2172–8.
Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, et al. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26(53):7445–56. doi:10.1038/sj.onc.1210546.
Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial–mesenchymal transition. Cancer Res. 2007;67(7):2922–6. doi:10.1158/0008-5472.CAN-06-3598.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi:10.1038/nrm1835.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58. doi:10.1016/j.ceb.2005.08.001.
Mo YY, Beck WT. Association of human DNA topoisomerase IIalpha with mitotic chromosomes in mammalian cells is independent of its catalytic activity. Exp Cell Res. 1999;252(1):50–62. doi:10.1006/excr.1999.4616.
Rieger-Christ KM, Lee P, Zagha R, Kosakowski M, Moinzadeh A, Stoffel J, et al. Novel expression of N-cadherin elicits in vitro bladder cell invasion via the Akt signaling pathway. Oncogene. 2004;23(27):4745–53. doi:10.1038/sj.onc.1207629.
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66(12):6225–32. doi:10.1158/0008-5472.CAN-06-0094.
Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602(2):151–61.
Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial–mesenchymal transition. J Cell Biol. 2005;168(1):29–33. doi:10.1083/jcb.200409067.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol. 2004;6(10):931–40. doi:10.1038/ncb1173.
Yamamura H, Ugawa S, Ueda T, Morita A, Shimada S. TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol Cell Physiol. 2008;295(2):C296–301. doi:10.1152/ajpcell.00499.2007.
Li Q, Wang X, Yang Z, Wang B, Li S. Menthol induces cell death via the TRPM8 channel in the human bladder cancer cell line T24. Oncology. 2009;77(6):335–41. doi:10.1159/000264627.
Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest. 2007;117(6):1647–57. doi:10.1172/JCI30168.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi:10.1038/35036035.
Weber CE, Li NY, Wai PY, Kuo PC. Epithelial–mesenchymal transition, TGF-beta, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res Off Publ Am Burn Assoc. 2012;33(3):311–8. doi:10.1097/BCR.0b013e318240541e.
Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(Pt 1):1–16.
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–9. doi:10.1038/35000034.
Katoh M. Epithelial–mesenchymal transition in gastric cancer (Review). Int J Oncol. 2005;27(6):1677–83.
Roxanis I. Occurrence and significance of epithelial–mesenchymal transition in breast cancer. J Clin Pathol. 2013;66(6):517–21. doi:10.1136/jclinpath-2012-201348.
Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol. 2008;221(1):1–6. doi:10.1007/s00232-007-9080-6.
Potier M, Joulin V, Roger S, Besson P, Jourdan ML, Leguennec JY, et al. Identification of SK3 channel as a new mediator of breast cancer cell migration. Mol Cancer Ther. 2006;5(11):2946–53. doi:10.1158/1535-7163.MCT-06-0194.
Zhang L, Zou W, Zhou SS, Chen DD. Potassium channels and proliferation and migration of breast cancer cells. Sheng li xue bao [Acta Physiol Sin]. 2009;61(1):15–20.
Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11(15):5381–9. doi:10.1158/1078-0432.CCR-05-0327.
Roger S, Potier M, Vandier C, Besson P, Le Guennec JY. Voltage-gated sodium channels: new targets in cancer therapy? Curr Pharm Des. 2006;12(28):3681–95.
Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15(2):124–34. doi:10.1016/j.ccr.2008.12.019.
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial–mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119(12):3626–36. doi:10.1172/JCI39374.
Slominski A. Cooling skin cancer: menthol inhibits melanoma growth. Focus on TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol Cell Physiol. 2008;295(2):C293–5. doi:10.1152/ajpcell.00312.2008.
Bai VU, Murthy S, Chinnakannu K, Muhletaler F, Tejwani S, Barrack ER, et al. Androgen regulated TRPM8 expression: a potential mRNA marker for metastatic prostate cancer detection in body fluids. Int J Oncol. 2010;36(2):443–50.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No.81272546).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liu and Chen contributed equally to this work
Rights and permissions
About this article
Cite this article
Liu, J., Chen, Y., Shuai, S. et al. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumor Biol. 35, 8969–8977 (2014). https://doi.org/10.1007/s13277-014-2077-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2077-8