Abstract
Backgrounds: Bee venom has been used as an alternative medicine for immune-related diseases such as multiple sclerosis (MS). Previously, we showed that Bee venom exerts a therapeutic effect in a mouse model of MS through regulatory T cells (Tregs) and Phospholipase A2 from bee venom (bvPLA2) induces a differentiation of Tregs.
Methods: To induce EAE, C57BL/6 mice were injected MOG35-55 peptide in CFA and pertussis toxin. To ascertain whether Tregs were involved in the neural protective effect of bvPLA2, the mice received a PC61 an-ti-CD25 mAb to deplete Tregs or normal anti-rat IgG1 for an isotype control. To verify the necessity of enzymatic activity experimentally, we synthesized a recombinant bvPLA2 and mutant bvPLA2, which has no catalytic ability.
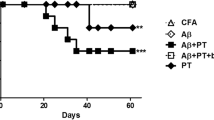
Results: The limb paralysis caused by EAE was significantly attenuated in the bvPLA2-treated mice compared to the PBS-treated mice. The beneficial effects of bvPLA2 disappeared when Tregs were depleted. Synthetic bvPLA2 showed therapeutic effects such as those of the natural bvPLA2; however, the mutant which has no catalytic ability did not.
Conclusion: Our findings suggest that bvPLA2 contributes to the control of MS and that its catalytic ability is needed for its therapeutic action.
Similar content being viewed by others
References
Steinman, L. Multiple sclerosis: a two-stage disease. Nat Immunol 2, 762–764 (2001).
Berer, K. & Krishnamoorthy, G. Microbial view of central nervous system autoimmunity. FEBS Lett 588, 4207–4213 (2014).
Global Burden of Disease Study, C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015).
Murray, T. J. Complementary and alternative medicine for MS. Int MS J 13, 3 (2006).
Castro, H. J. et al. A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy and Asthma Proc 26, 470–476 (2005).
Lee, J. D., Park, H. J., Chae, Y. & Lim, S. An Overview of Bee Venom Acupuncture in the Treatment of Arthritis. Evid Based Complement Alternat Med 2, 79–84 (2005).
Liu, X. D. et al. Clinical randomized study of bee-sting therapy for rheumatoid arthritis. Zhen Ci Yan Jiu 33, 197–200 (2008).
Walker, E. W. Bees’ Stings and Rheumatism. Br Med J 2, 1056–1060 (1908).
Maberly, F H. Brief notes in the treatment of rheumatism by bee stings. Lancet 2, 235 (1910).
Billingham, M. E. et al. Letter: An anti-inflammatory peptide from bee venom. Nature 245, 163–164 (1973).
Orlov, B. N. & Romanova, E. B. Regulatory action of bee venom on the humoral immunity system. Nau-chnye Doli Vyss Shkoly Biol Nauki 4, 41–45 (1982).
Hider, R. C. Honeybee venom: a rich source of pharmacologically active peptides. Endeavour 12, 60–65 (1988).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Choi, M. S. et al. Bee venom ameliorates ovalbumin induced allergic asthma via modulating CD4+CD25+ regulatory T cells in mice. Cytokine 61, 256–265 (2013).
Lee, G. et al. Bee Venom Attenuates Experimental Autoimmune Encephalomyelitis through Direct Effets on Cd4 (+)Cd25 (+)Foxp3 (+) T Cells. Eur J Inflamm 11, 111–121 (2013).
Chung, E. S. et al. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson’s disease: role of regulatory T cells. Brain Behav Immun 26, 1322–1330 (2012).
Chung, E. S. et al. Bee Venom Phospholipase A2, a Novel Foxp3+ Regulatory T Cell Inducer, Protects Dopaminergic Neurons by Modulating Neuroinflammatory Responses in a Mouse Model of Parkinson’s Disease. J Immunol 195, 4853–4860 (2015).
Shin, D. et al. Regulatory T Cells Contribute to the Inhibition of Radiation-Induced Acute Lung Inflammation via Bee Venom Phospholipase A(2) in Mce. Toxins (Basel) 8, 5 (2016).
Park, S. et al. Bee venom phospholipase A2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory T cells. Immun Inflamm Dis 3, 386–397 (2015).
Ye, M. et al. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J Neuroinflammation 13, 10 (2016).
Lee, G. & Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins (Basel) 8, 48 (2016).
Dudler, T. et al. High-level expression in Escherichia coli and rapid purification of enzymatically active honey bee venom phospholipase A2. Biochim Biophys Acta 1165, 201–210 (1992).
Natarajan, C. & Bright, J. J. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol 168, 6506–6513 (2002).
Constantinescu, C. S., Farooqi, N., O’ Brien, K. & Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164, 1079–1106 (2011).
Kwon, Y. B. et al. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life SCi 71, 191–204 (2002).
Eiseman, J. L., von Bredow, J. & Alvares, A. P. Effect of honeybee (Apis mellifera) venom on the course of adjuvantinduced arthritis and depression of drug metabolism in the rat. Biochem Pharmacol 31, 1139–1146 (1982).
Koutrolos, M. et al. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun 2, 163 (2014).
Akirav, E. M., Bergman, C. M., Hill, M. & Ruddle, N. H. Depletion of CD4 (+)CD25 (+) T cells exacerbates experimental autoimmune encephalomyelitis induced by mouse, but not rat, antigens. J Neurosci Res 87, 3511–3519 (2009).
Jager, A. et al. Th1, Thl7, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 183, 7169–7177 (2009).
Baek, H. et al. Bee venom phospholipase A2 ameliorates Alzheimer’s disease pathology in Abeta vaccination treatment without inducing neuro-inflammation in a 3xTg-AD mouse model. SCi Rep 8, 17369 (2018).
Jung, K. H. et al. Bee Venom Phospholipase A2 Ameliorates House Dust Mite Extract Induced Atopic Dermatitis Like Skin Lesions in Mce. Toxins (Basel) 9, 2 (2017).
Palm, N. W. et al. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 39, 976–985 (2013).
Acknowledgements
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2017R1A2B3009 574).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
Gihyun Lee, Geun-Hyung Kang & Hyunsu Bae declares that they have no conflict of interest.
Human and animal rights
All studies with animals were performed in accordance with the approved animal protocol and guidelines established by the Animal Care and Use Committee of Kyung Hee University. The article does not contain any studies with human.
Rights and permissions
About this article
Cite this article
Lee, G., Kang, GH. & Bae, H. Bee venom phospholipase A2 suppression of experimental autoimmune encephalomyelitis is dependent on its enzymatic activity. Mol. Cell. Toxicol. 15, 307–313 (2019). https://doi.org/10.1007/s13273-019-0034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-019-0034-8