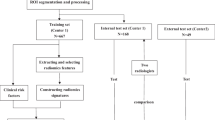
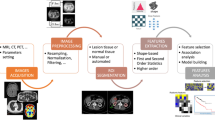
Abstract
A classifier-based expert system was developed to compare delivered and planned radiation therapy in prostate cancer patients. Its aim is to automatically identify patients that can benefit from an adaptive treatment strategy. The study predominantly addresses dosimetric uncertainties and critical issues caused by motion of hollow organs. 1200 MVCT images of 38 prostate adenocarcinoma cases were analyzed. An automatic daily re-contouring of structures (i.e. rectum, bladder and femoral heads), rigid/deformable registration and dose warping was carried out to simulate dose and volume variations during therapy. Support vector machine, K-means clustering algorithms and similarity index analysis were used to create an unsupervised predictive tool to detect incorrect setup and/or morphological changes as a consequence of inadequate patient preparation due to stochastic physiological changes, supporting clinical decision-making. After training on a dataset that was considered sufficiently dosimetrically stable, the system identified two equally sized macro clusters with distinctly different volumetric and dosimetric baseline properties and defined thresholds for these two clusters. Application to the test cohort resulted in 25% of the patients located outside the two macro clusters thresholds and which were therefore suspected to be dosimetrically unstable. In these patients, over the treatment course, mean volumetric changes of 30 and 40% for rectum and bladder were detected which possibly represents values justifying adjustment of patient preparation, frequent re-planning or a plan-of-the-day strategy. Based on our research, by combining daily IGRT images with rigid/deformable registration and dose warping, it is possible to apply a machine learning approach to the clinical setting obtaining useful information for a decision regarding an individualized adaptive strategy. Especially for treatments influenced by the movement of hollow organs, this could reduce inadequate treatments and possibly reduce toxicity, thereby increasing overall RT efficacy.










Similar content being viewed by others
References
Cinar M, Engin M, Engin E Z, Atesci Y Z (2009) Early prostate cancer diagnosis by using artificial neural networks and support vector machines. Expert Syst Appl 36: 6357–6361
Murena LP, Ekerolda R, Kvinnsland Y, Karlsdottir A, Dahl O (2004) On the use of margins for geometrical uncertainties around the rectum in radiotherapy planning. Radiother Oncol 70:11–19
Voyant C, Biffi K, Leschi D, Briançon J, Lantieri C (2011) Dosimetric uncertainties related to the elasticity of bladder and rectal walls: adenocarcinoma of the prostate. Cancer/Radiothérapie 15: 270–278
Piotrowski T, Yartsev S, Rodrigues G, Bajon T (2014) Comparative analysis of image guidance in two institutions for prostate cancer patients. Rep Pract Oncol Radiother 19:206–213
Skorska M, Piotrowski T (2013) Empirical estimation of beam-on time for prostate cancer patients treated on tomotherapy. Rep Pract Oncol Radiother 18:201–208
Fiorino C, Di Muzio N, Broggi S, Cozzarini C, Maggiulli E, Alongi F et al (2008) Evidence of limited motion of the prostate by carefully emptying the rectum as assessed by daily MVCT image guidance with helical tomotherapy. Int J Radiat Oncol Biol Phys 71:611–617
Buchali A, Koswig S, Dinges S, Rosenthal P, Salk J, Lackner G et al (1999) Impact of the filling status of the bladder and rectum on their integral dose distribution and the movement of the uterus in the treatment planning of gynaecological cancer. Radiother Oncol 52:29–34
Fiorino C, Rancati T, Fellin G, Vavassori V, Cagna E, Borca VC et al (2012) Late fecal incontinence after high-dose radiotherapy for prostate cancer: better prediction using longitudinal definitions. Int J Radiat Oncol Biol Phys 83:38–45
Fokdal L, Honoré H, Høyer M, Meldgaard P, Fode K, von der Maase H (2004) Impact of changes in bladder and rectal filling volume on organ motion and dose distribution of the bladder in radiotherapy for urinary bladder cancer. Int J Radiat Oncol Biol Phys 59:436–444
Valdagni R, Kattan MW, Rancati T, Yu C, Vavassori V, Fellin G et al (2012) Is it time to tailor the prediction of radio-induced toxicity in prostate cancer patients? Building the first set of nomograms for late rectal syndrome. Int J Radiat Oncol Biol Phys 82:1957–1966
Dawson L, Litzenberg W, Brock KK, Sanda M, Sullivan M, Sandler HM et al (2000) A comparison of ventilatory prostate movement in four treatment positions. Int J Radiat Oncol Biol Phys 48:319–323
Nichol A, Brock KK, Lockwood GA, Math M, Moseley DJ, Rosewall T et al (2007) A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys 67:48–56
Adamczyk M, Piotrowski T, Adamiak E (2012) Evaluation of combining bony anatomy and soft tissue position correction strategies for IMRT prostate cancer patients. Rep Pract Oncol Radiother 17:104–109
Ogino I, Uemura H, Inoue T, Kubota Y, Nomura K, Okamoto N (2008) Reduction of prostate motion by removal of gas in rectum during radiotherapy. Int J Radiat Oncol Biol Phys 72:456–466
Yamashita H, Takenaka R, Sakumi A, Haga A, Otomo K, Nakagawa K (2015) Analysis of motion of the rectum during preoperative intensity modulated radiation therapy for rectal cancer using cone-beam computed tomography. Radiat Oncol 10:1–7
Van Herk M, Bruce A, Kroes G, Shouman T, Touw A, Lebesque JV (2005) Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int J Radiat Oncol Biol Phys 33:1311–1320
Olteanu L A M, Madani I, De Neve W, Vercauteren T, De Gersem W (2012) Evaluation of deformable image coregistration in adaptive dose painting by numbers for head and neck cancer. Int J Radiat Oncol Biol Phys 83:696–703
Lee C, Langeon KM, Lu W, Schnarr E, Ruchala KJ, Olivera GH et al (2008) Evaluation of geometric changes of parotid glands during head and neck cancer radiotherapy using daily MVCT and automatic deformable registration. Radiother Oncol 89:81–88
Reddy NM, Nori D, Sartin W, Maiorano S, Modena J, Mazur A et al (2009) Influence of volumes of prostate, rectum, and bladder on treatment planning CT on interfraction prostate shifts during ultrasound image guided IMRT. Med Phys 36:11
Lutkenhaus L, Visser J, de Jong R, Hulshof M, Bel A (2015) Evaluation of delivered dose for a clinical daily adaptive plan selection strategy for bladder cancer radiotherapy. Radiother Oncol 116:51–56
Guidi G, Maffei N, Vecchi C, Ciarmatori A, Mistretta G M, Gottardi G et al (2015) A support vector machine tool for adaptive tomotherapy treatments: prediction of head and neck patients criticalities. Phys Med 31: 442–451
Balter JM, Sandler HM, Lam K, Bree RL, Lichter AS, ten Haken RK (1995) Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys 31:113–118
Velker V, Louiel AV, Markham J, Rodrigues GB (2012) Predictors of prostate bed contouring variability: an international contouring challenge. Int J Radiat Oncol Biol Phys 84:362–363
Brock KK (2010) Results of a multi-institution deformable registration accuracy study (midras). Int J Radiat Oncol Biol Phys 76:583–596
Weistrand O, Svensson S (2015) The ANACONDA algorithm for deformable image registration in radiotherapy. Med Phys 42:40–53
Spoerka J, Gendrina C, Webera C, Figl M, Pawiro SA, Furtado H et al (2012) High-performance GPU-based rendering for real-time, rigid 2D/3D-image registration and motion prediction in radiation oncology. Z Med Phys 22:13–20
Ziegenhein P, Pirner S, Kamerling C, Oelfke U (2015) Fast CPU-based Monte Carlo simulation for radiotherapy dose calculation. Phys Med Biol 60:6097–6111
Kononenko I (2001) Machine learning for medical diagnosis: history, state of the art and perspective. Artif Intell Med 23:89–109
Bengio Y, Glorot X (2010) Understanding the difficulty of training deep feed forward neural networks. AISTATS 9:249–256
Larochelle H, Bengio Y, Louradour J, Lamblin P (2009) Exploring strategies for training deep neural networks. JMLR 1: 1–40
Gulliford SL, Webba S, Rowbottom CG, Corne DW, Dearnaley DP (2004) Use of artificial neural networks to predict biological outcomes for patients receiving radical radiotherapy of the prostate. Radiother Oncol 71:3–12
Du KL (2010) Clustering: a neural network approach. Neural Netw 23:89–107
Lu L, Jin C, Zhou T (2009) Effective and efficient similarity index for link prediction of complex networks. Phys Rev 80:046122
Sawaa T, Ohno-Machadoa L (2003) A neural network-based similarity index for clustering DNA microarray data. Comput Biol Med 33:1–15
Fränti P, Rezaei M, Zhao Q (2014) Centroid index: cluster level similarity measure. Pattern Recogn 47:3034–3045
Zhang Q, Shang M, Zeng W, Chen Y, Lu L (2010) Empirical comparison of local structural similarity indices for collaborative-filtering-based recommender systems. Phys Proc 3: 1887–1896
Albatineh AN (2010) Means and variances for a family of similarity indices used in cluster analysis. J Stat Plan Infer 140:2828–2838
Alqadah F, Bhatnagar R (2011) Similarity measures in formal concept analysis. Ann Math Artif Intell 61:245–256
Chao A, Chazdon RL, Colwell RK, Shen TJ (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159
Chao A, Chazdon RL, Colwell RK, Shen TJ (2006) Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 62:361–371
Boyce RL, Ellison PC (2001) Choosing the best similarity index when performing fuzzy set ordination on binary data. J Veg Sci 12:711–720
Deasy JO, Niemierko A, Herbert D, Yan D, Jackson A, Ten Haken RK, Langer M, Sapareto S (2002) Methodological issues in radiation dose-volume outcome analyses: summary of a joint AAPM/NIH workshop. Med Phys 29(9):2109–2127
Barker JL, Garden AS, Ang KK, O’Daniel JC, Wang H, Court L et al (2004) Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head and neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 59:960–970
Nishi T, Nishimura Y, Shibata T, Tamura M, Nishigaito N, Okumura M (2013) Volume and dosimetric changes and initial clinical experience of a two-step adaptive intensity modulated radiation therapy (IMRT) scheme for head and neck cancer. Radiother Oncol 106:85–89
Maffei N, Guidi G, Vecchi C, Baldazzi G, Costi T (2014) predictive neural network for parotid glands deformation using IGRT and dose warping systems. Med Phys 41:177
Murena LP, Smaalanda R, Dahl O (2003) Organ motion, set-up variation and treatment margins in radical radiotherapy of urinary bladder cancer. Radiother Oncol 69:291–304
Yadav P, Ramasubramanian V, Paliwal BR (2011) Feasibility study on effect and stability of adaptive radiotherapy on kilovoltage cone beam CT. Radiol Oncol 45(3):220–226
Yadav P, Tolakanahalli R, Rong Y, Paliwal BR (2010) The effect and stability of MVCT images on adaptive TomoTherapy. J Appl Clin Med Phys 11(4):3229
Acknowledgements
The research has been developed at Medical Physics and Radiation Oncology Department of Az. Ospedaliero-Universitaria of Modena inside a research project partially funded by the Italian Ministry of Health and an industrial partnership: Italian Research Grant N. MoH (GR-2010-2318757) “Dose warping methods for IGRT and Adaptive RT: dose accumulation based on organ motion and anatomical variations of the patients during radiation therapy treatments”; Tecnologie Avanzate S.r.l. (Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All the authors gave consent before inclusion.
Rights and permissions
About this article
Cite this article
Guidi, G., Maffei, N., Vecchi, C. et al. Expert system classifier for adaptive radiation therapy in prostate cancer. Australas Phys Eng Sci Med 40, 337–348 (2017). https://doi.org/10.1007/s13246-017-0535-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-017-0535-5