Abstract
Purpose
Bioprosthetic Heart Valves (BHVs) are widely used in clinical practice, showing promising outcomes. Computational modeling offers a valuable tool for quantitatively characterizing BHVs. To ensure the accuracy of computational models, it is crucial to consider precise leaflet properties, including mechanical properties and density. Bovine pericardium (BP) serves as a common material for BHV leaflets. Previous computational studies often assume BP density to approximate that of water or blood. Given that BP leaflets undergo various treatments, such as tissue fixation and anti-calcification, this study aims to measure the density of BP used in BHVs and assess its impact on leaflet stress distribution.
Methods
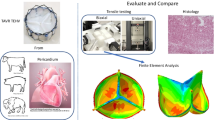
Eight square BP samples were laser cut from Edwards BP patches and their density was determined. Specimen weight was measured using an A&D Analytical Balance, while volume was assessed through high-resolution imaging. Additionally, finite element models resembling a BHV, like the Carpentier-Edwards PERIMOUNT Magna, were constructed in ABAQUS.
Results
The average density of the BP samples was found to be 1,410 kg/m3. During the acceleration phase of a cardiac cycle, the maximum stress reached 1.89 MPa for a density of 1,410 kg/m3 and 2.47 MPa for a density of 1,000 kg/m3 (a 30.7% difference). In the deceleration phase, the maximum stress reached 713 kPa and 669 kPa, respectively.
Conclusion
Leaflet stress distribution and motion in BHVs are influenced by density variations. Establishing an accurate density value for BHV leaflets is imperative for enhancing the computational models, which can ultimately contribute to improved BHV design and outcomes.







Similar content being viewed by others
References
Mohammadi, H., and K. Mequanint. Prosthetic aortic heart valves: modeling and design. Mech. Eng. Phys.. 33(2):131–147, 2011.
Schoen, F. J. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 118(18):1864–1880, 2008.
Tam, H., et al. Fixation of bovine pericardium-based tissue biomaterial with irreversible chemistry improves biochemical and biomechanical properties. J. Cardiovasc. Transl. Res.. 10:194–205, 2017.
Soares, J. S., et al. Biomechanical behavior of bioprosthetic heart valve heterograft tissues: characterization, simulation, and performance. Cardiovas. Eng. Technol.. 7:309–351, 2016.
Hiester, E.D. and M.S. Sacks. Optimal bovine pericardial tissue selection sites. I. Fiber architecture and tissue thickness measurements. J. Biomed. Mater. Res. 39(2): 207–214, 1998.
Cunanan, C. M., et al. Tissue characterization and calcification potential of commercial bioprosthetic heart valves. Ann. Thorac. Surg.. 71(5):S417–S421, 2001.
Dove, J., M. Howanec, and M. Thubrikar, Carpentier-Edwards ThermaFix Process: a method for extracting calcium binding sites from pericardial tissue. Edwards Lifesciences LLC, 2006.
Flameng, W., et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J. Thorac. Cardiovasc. Surg.. 149(1):340–345, 2015.
Puskas, J. D., et al. The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur. J. Cardio-Thorac. Surg.. 52(3):432–439, 2017.
Johnston, D. R., et al. Intermediate-term outcomes of aortic valve replacement using a bioprosthesis with a novel tissue. J. Thorac. Cardiovasc. Surg.. 162(5):1478–1485, 2021.
Sun, W., A. Abad, and M.S. Sacks, Simulated bioprosthetic heart valve deformation under quasi-static loading. 2005.
Sacks, M. S., et al. Bioprosthetic heart valve heterograft biomaterials: structure, mechanical behavior and computational simulation. Expert Rev. Med. Dev.. 3(6):817–834, 2006.
Sun, W., et al. Biaxial mechanical response of bioprosthetic heart valve biomaterials to high in-plane shear. J. Biomed. eng.. 125(3):372–380, 2003.
Borazjani, I. Fluid-structure interaction, immersed boundary-finite element method simulations of bio-prosthetic heart valves. Comput. Methods Appl. Mech.Eng.. 257:103–116, 2013.
Abbasi, M., and A. N. Azadani. Leaflet stress and strain distributions following incomplete transcatheter aortic valve expansion. J. Biomech.. 48(13):3663–3671, 2015.
Abbasi, M., et al. Characterization of three-dimensional anisotropic heart valve tissue mechanical properties using inverse finite element analysis. J. Mech. Behav. Biomed. Materi. 62:33–44, 2016.
Association, G.P., Physical properties of glycerine and its solutions. 1963: Glycerine Producers' Association.
Abbasi, M., et al. A non-invasive material characterization framework for bioprosthetic heart valves. Ann. Biomed. Eng.. 47:97–112, 2019.
Kim, H., et al., Dynamic simulation pericardial bioprosthetic heart valve function. 2006.
Vesely, I. The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovasc. Pathol.. 12(5):277–286, 2003.
Gauvin, R., et al. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J. Biomed. Appl.. 28(4):552–565, 2013.
Schoen, F. J., and R. J. Levy. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann. Thorac. Surg.. 79(3):1072–1080, 2005.
Bourguignon, T., et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann. Thorac. Surg.. 99(3):831–837, 2015.
Søndergaard, L., et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J. Am. College Cardiol.. 73(5):546–553, 2019.
Campion, G., et al. A biomechanical and microstructural analysis of bovine and porcine pericardium for use in bioprosthetic heart valves. Struct. Heart. 5(5):486–496, 2021.
Caballero, A., et al. Evaluation of transcatheter heart valve biomaterials: Biomechanical characterization of bovine and porcine pericardium. J. Mech. Behav. Biomed.materials. 75:486–494, 2017.
Oswal, D., et al. Biomechanical characterization of decellularized and cross-linked bovine pericardium. J. Heart Valve Dis.. 16(2):165, 2007.
Funding
This work was supported by the University of Denver (Grant No.: 84993-142235).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadipour, M., Azadani, A.N. The Measurement of Bovine Pericardium Density and Its Implications on Leaflet Stress Distribution in Bioprosthetic Heart Valves. Cardiovasc Eng Tech 14, 853–861 (2023). https://doi.org/10.1007/s13239-023-00692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-023-00692-0