Abstract
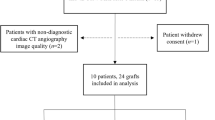
Cardiac allograft vasculopathy (CAV) is one of the leading causes of morbidity and morality in orthotopic heart transplant (HTx) patients. While disturbed flow patterns have been linked to the spatial localization of atherosclerosis, the role of hemodynamics in CAV development has not been examined. HTx patients (n = 5) requiring percutaneous coronary intervention (PCI) for a focal, epicardial lesion were studied. Angiographic images were retrospectively obtained from baseline (i.e., in the presence of no observed disease) and follow-up catheterizations (i.e., at the time of PCI; 12.4 ± 2.6 years post-HTx). Patient-specific computational models were created from baseline images. Computational fluid dynamic techniques were employed to quantify the hemodynamic environment, which was expressed as normalized time-averaged WSS (TAWSSnorm; measure of temporal WSS magnitude) and normalized WSS angle deviation (WSSADnorm; measure of instantaneous WSS vector oscillation) values. Baseline hemodynamic and follow-up angiographic data were co-registered to investigate the association between WSS and subsequent occlusive CAV lesion location. Results indicate a high degree of co-localization between baseline low WSS data and follow-up occlusive CAV lesion. Local minima in TAWSSnorm were located 2.5 ± 0.6 mm from the site of PCI. Furthermore, local maxima in WSSADnorm were located 3.9 ± 0.7 mm from the site of PCI. In 3 patients, the occlusive lesion formed in a region that was subjected to both low and oscillatory WSS at baseline. There was discernable spatial co-localization between baseline disturbed flow patterns and follow-up CAV lesions requiring PCI. These results suggest a role of fluid mechanics in the development of focal, flow-limiting CAV lesions.




Similar content being viewed by others
References
Billingham, M. E. Cardiac transplant atherosclerosis. Transpl. Proc. 19(4 Suppl 5):19–25, 1987.
Billingham, M. E. Histopathology of graft coronary disease. J. Heart Lung Transpl. 11(3 Pt 2):S38–S44, 1992.
Braith, R. W., R. M. Mills, C. S. Wilcox, G. L. Davis, J. A. Hill, and C. E. Wood. High-dose angiotensin-converting enzyme inhibition restores body fluid homeostasis in heart-transplant recipients. J. Am. Coll. Cardiol. 41(3):426–432, 2003.
Buchanan, J. R., C. Kleinstreuer, S. Hyun, and G. A. Truskey. Hemodynamics simulation and identification of susceptible sites of atherosclerotic lesion formation in a model abdominal aorta. J. Biomech. 36(8):1185–1196, 2003.
Cheng, C., D. Tempel, R. van Haperen, A. van der Baan, F. Grosveld, M. J. Daemen, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113(23):2744–2753, 2006. doi:10.1161/CIRCULATIONAHA.105.590018.
Chiu, J. J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91(1):327–387, 2011. doi:10.1152/physrev.00047.2009.
Dart, A. M., F. Lacombe, J. K. Yeoh, J. D. Cameron, G. L. Jennings, E. Laufer, et al. Aortic distensibility in patients with isolated hypercholesterolaemia, coronary artery disease, or cardiac transplant. Lancet 338(8762):270–273, 1991.
El-Hamamsy, I., L. M. Stevens, P. M. Vanhoutte, and L. P. Perrault. Injury of the coronary endothelium at implantation increases endothelial dysfunction and intimal hyperplasia after heart transplantation. J. Heart Lung Transpl. 24(3):251–258, 2005. doi:10.1016/j.healun.2003.12.012.
Goubergrits, L., E. Wellnhofer, U. Kertzscher, K. Affeld, C. Petz, and H. C. Hege. Coronary artery WSS profiling using a geometry reconstruction based on biplane angiography. Ann. Biomed. Eng. 37(4):682–691, 2009. doi:10.1007/s10439-009-9656-7.
Haddad, M., P. W. Pflugfelder, C. Guiraudon, R. J. Novick, F. N. McKenzie, A. Menkis, et al. Angiographic, pathologic, and clinical relationships in coronary artery disease in cardiac allografts. J. Heart Lung Transpl. 24(9):1218–1225, 2005. doi:10.1016/j.healun.2004.08.016.
He, X., and D. N. Ku. Pulsatile flow in the human left coronary artery bifurcation: average conditions. J. Biomech. Eng. 118(1):74–82, 1996.
Humphrey, J. D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York, NY: Springer-Verlag, 2002.
Humphrey, J. D. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52(2):195–200, 2008. doi:10.1161/HYPERTENSIONAHA.107.103440.
Intengan, H. D., and E. L. Schiffrin. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38(3 Pt 2):581–587, 2001.
Kass, M., R. Allan, and H. Haddad. Diagnosis of graft coronary artery disease. Curr. Opin. Cardiol. 22(2):139–145, 2007. doi:10.1097/HCO.0b013e328021066b.
Kobashigawa, J. A., J. M. Tobis, R. C. Starling, E. M. Tuzcu, A. L. Smith, H. A. Valantine, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J. Am. Coll. Cardiol. 45(9):1532–1537, 2005. doi:10.1016/j.jacc.2005.02.035.
Konig, A., E. Kilian, H. Y. Sohn, J. Rieber, T. M. Schiele, U. Siebert, et al. Assessment and characterization of time-related differences in plaque composition by intravascular ultrasound-derived radiofrequency analysis in heart transplant recipients. J. Heart Lung Transpl. 27(3):302–309, 2008. doi:10.1016/j.healun.2007.12.003.
Krams, R., J. J. Wentzel, J. A. Oomen, R. Vinke, J. C. Schuurbiers, P. J. de Feyter et al. Evaluation of endothelial shear stress and 3D geometry as factors determining the development of atherosclerosis and remodeling in human coronary arteries in vivo. Combining 3D reconstruction from angiography and IVUS (ANGUS) with computational fluid dynamics. Arteriosclerosis, Thrombosis, and Vascular Biology 17(10):2061–2065, 1997.
Ku, D. N. Blood flow in arteries. Annu. Rev. Fluid Mech. 29:399–434, 1997. doi:10.1146/Annurev.Fluid.29.1.399.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5(3):293–302, 1985.
Longest, P. W., and C. Kleinstreuer. Computational haemodynamics analysis and comparison study of arterio-venous grafts. J. Med. Eng. Technol. 24(3):102–110, 2000.
Matsumoto, T., and K. Hayashi. Mechanical and dimensional adaptation of rat aorta to hypertension. J. Biomech. Eng. 116(3):278–283, 1994.
Matsuzaki, I., S. Chatterjee, K. Debolt, Y. Manevich, Q. Zhang, and A. B. Fisher. Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 288(1):H336–H343, 2005. doi:10.1152/ajpheart.00025.2004.
Mintz, G. S., S. E. Nissen, W. D. Anderson, S. R. Bailey, R. Erbel, P. J. Fitzgerald et al.: American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. Journal of the American College of Cardiology. 2001;37(5):1478-92.
Moore, Jr., J. E., C. Xu, S. Glagov, C. K. Zarins, and D. N. Ku. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110(2):225–240, 1994.
Myers, B. D., R. Sibley, L. Newton, S. J. Tomlanovich, C. Boshkos, E. Stinson, et al. The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int. 33(2):590–600, 1988.
Nam, D., C. W. Ni, A. Rezvan, J. Suo, K. Budzyn, A. Llanos, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297(4):H1535–H1543, 2009. doi:10.1152/ajpheart.00510.2009.
Pethig, K., T. Kofidis, B. Heublein, A. Westphal, and A. Haverich. Impact of vascular branching sites on focal progression of allograft vasculopathy in transplanted hearts. Atherosclerosis 158(1):155–160, 2001.
Pucci, A. M., R. D. Forbes, and M. E. Billingham. Pathologic features in long-term cardiac allografts. J. Heart Lung Transpl. 9(4):339–345, 1990.
Rahmani, M., R. P. Cruz, D. J. Granville, and B. M. McManus. Allograft vasculopathy versus atherosclerosis. Circ. Res. 99(8):801–815, 2006. doi:10.1161/01.RES.0000246086.93555.f3.
Samady, H., P. Eshtehardi, M. C. McDaniel, J. Suo, S. S. Dhawan, C. Maynard, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 124(7):779–788, 2011. doi:10.1161/CIRCULATIONAHA.111.021824.
Scherrer, U., S. F. Vissing, B. J. Morgan, J. A. Rollins, R. S. Tindall, S. Ring, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N. Engl. J. Med. 323(11):693–699, 1990. doi:10.1056/NEJM199009133231101.
Shi, Z. D., and J. M. Tarbell. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann. Biomed. Eng. 39(6):1608–1619, 2011. doi:10.1007/s10439-011-0309-2.
Starling, R. C., and R. J. Cody. Cardiac transplant hypertension. Am. J. Cardiol. 65(1):106–111, 1990.
Stehlik, J., L. B. Edwards, A. Y. Kucheryavaya, C. Benden, J. D. Christie, A. I. Dipchand, et al. The registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report–2012. J. Heart Lung Transpl. 31(10):1052–1064, 2012. doi:10.1016/j.healun.2012.08.002.
Stone, P. H., S. Saito, S. Takahashi, Y. Makita, S. Nakamura, T. Kawasaki, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation 126(2):172–181, 2012. doi:10.1161/CIRCULATIONAHA.112.096438.
Suo, J., D. E. Ferrara, D. Sorescu, R. E. Guldberg, W. R. Taylor, and D. P. Giddens. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler. Thromb. Vasc. Biol. 27(2):346–351, 2007. doi:10.1161/01.ATV.0000253492.45717.46.
Syeda, B., S. Roedler, C. Schukro, N. Yahya, A. Zuckermann, and D. Glogar. Transplant coronary artery disease: incidence, progression and interventional revascularization. Int. J. Cardiol. 104(3):269–274, 2005. doi:10.1016/j.ijcard.2004.10.033.
Tanaka, M., G. K. Mokhtari, R. D. Terry, L. B. Balsam, K. H. Lee, T. Kofidis et al. Overexpression of human copper/zinc superoxide dismutase (SOD1) suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts. Circulation 110(11 Suppl 1):II200-6, 2004. doi:10.1161/01.CIR.0000138390.81640.54.
Timmins, L. H., J. Suo, P. Eshtehardi, S. S. Dhawan, M. C. McDaniel, J. N. Oshinski, et al., editors. Geometric and Hemodynamic Evaluation of 3-Dimensional Reconstruction Techniques for the Assessment of Coronary Artery Wall Shear Stress in the Setting of Clinical Disease Progression. ASME Summer Bioengineering Conference; June 2011; Nemacolin Woodlands Resort, Famington, PA.
Timmins, L. H., B. D. Mackie, J. N. Oshinski, D. P. Giddens, and H. Samady. Colocalization of low and oscillatory coronary wall shear stress with subsequent culprit lesion resulting in myocardial infarction in an orthotopic heart transplant patient. JACC Cardiovasc. Interv. 6(11):1210–1211, 2013. doi:10.1016/j.jcin.2013.03.024.
Tuzcu, E. M., S. R. Kapadia, R. Sachar, K. M. Ziada, T. D. Crowe, J. Feng, et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J. Am. Coll. Cardiol. 45(9):1538–1542, 2005. doi:10.1016/j.jacc.2004.12.076.
Wellnhofer, E., W. Bocksch, N. Hiemann, M. Dandel, W. Klimek, R. Hetzer, et al. Shear stress and vascular remodeling: study of cardiac allograft coronary artery disease as a model of diffuse atherosclerosis. J. Heart Lung Transpl. 21(4):405–416, 2002.
Weydahl, E. S., and J. E. Moore. Dynamic curvature strongly affects wall shear rates in a coronary artery bifurcation model. J. Biomech. 34(9):1189–1196, 2001.
Wood, K. J., and R. Goto. Mechanisms of rejection: current perspectives. Transplantation 93(1):1–10, 2012. doi:10.1097/TP.0b013e31823cab44.
Zeng, D., Z. Ding, M. H. Friedman, and C. R. Ethier. Effects of cardiac motion on right coronary artery hemodynamics. Ann. Biomed. Eng. 31(4):420–429, 2003.
Acknowledgments
Funding for this research was provided by American Heart Association (AHA) Postdoctoral Fellowships (to L. H. T. and D. S. M.) and the Georgia Research Alliance (to D. P. G.).
Human Studies/Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation as mandated by the Institutional Review Board at Emory University. Due to the retrospective nature of this investigation, informed consent was not required for the patients examined, and all data utilized was acquired for clinical reasons (i.e., these data were not investigational).
Animal studies
No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material
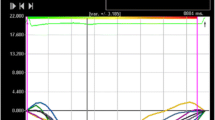
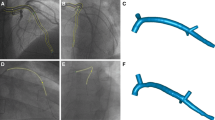
Angiographic reconstruction data were exported from the reconstruction software (IC-PRO, Paieon, Inc., Rosh Ha’ayin, Israel) as 3D Cartesian coordinates of the centerline and a corresponding diameter at each centerline point. Data were reported at increments of 0.25 mm along the vessel centerline, which is the approximate resolution of the angiographic images (ranged from 0.21 – 0.25 mm/pixel for patient cohort). As a result of the resolution of the imaging modality, and since the reconstructed artery boundary must be defined as an entire pixel (Supplemental Fig. 1A insert), small changes in artery diameter occurred in local regions. As a result, small oscillations in diameter data were observed along the length of the artery (Supplemental Fig. 1B, black curve). For example, in Supplemental Fig. IB between the centerline axial position of 33 – 37 mm, there is a noticeable oscillation with an amplitude of ~0.25 mm in the diameter. This amplitude value corresponds precisely to the resolution of the imaging data and is likely a limitation of the reconstruction technique. These oscillations are non-physiologic and strongly affect the resulting hemodynamic data as WSS is inversely related to the cube of the artery radius. For example, the artificial oscillations results in a banded appearance of the TAWSSnorm distributions (Supplemental Fig. 1D) as well as the analysis of the hemodynamics parameters along the major vessel of interest (Supplemental Fig. 1E).
To alleviate any non-anatomical artifacts on the reconstructed geometries, a smoothing algorithm was applied to the reconstruction data prior to computational model construction and CFD. Reconstructed centerline data were undersampled (i.e., smoothed) by extracting data points every 1.25 mm along the artery axes (main vessel and branches) and applying a weighted linear least squares regression smoothing algorithm. As a result, the previously observed oscillations were removed, while still preserving the anatomical shape of the artery (Supplemental Fig. 1B, C). Furthermore, the banded appearance of the resulting non-smoothed TAWSSnorm distributions was removed, yet the global hemodynamic environment was not significantly altered (Supplemental Fig. 1D, E). The smoothing algorithm still allowed for the accurate co-localization of disturbed baseline disturbed flow patterns and follow-up CAV lesion formation, and likely more accurately represented the anatomic physical system.
Supplement Fig. 1 Caption
Undersampling (smoothing) Algorithm of Angiographic Reconstructions. (A) Single-plane angiographic images with magnified insert to highlight the oscillations (ripples) in the diameter. (B) Average diameter along vessel main axis for unsmoothed and smoothed data. Note the oscillations in the unsmoothed diameter over small axial lengths. Vertical dotted lines denote location of side branches. (C) Reconstructed 3D geometries. (D) TAWSSnorm distributions. (E) TAWSSnorm values along main axis of vessel.
Rights and permissions
About this article
Cite this article
Timmins, L.H., Gupta, D., Corban, M.T. et al. Co-localization of Disturbed Flow Patterns and Occlusive Cardiac Allograft Vasculopathy Lesion Formation in Heart Transplant Patients. Cardiovasc Eng Tech 6, 25–35 (2015). https://doi.org/10.1007/s13239-014-0198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-014-0198-2