Abstract
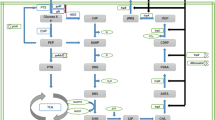
Acetate is a primary inhibitory metabolite in cultures of Escherichia coli, and the production of both biomass and desired products are increased by reducing the accumulation of acetate. In this study, the accumulation of acetate during ʟ-tryptophan production was decreased by genetic modification of ʟ-tryptophan-producing strain (BCTRP) and optimization of the fermentation process. The mutant (BCTRPG), which has a deletion of the integral membrane permease IICBGlc (ptsG), produces a higher concentration of ʟ-tryptophan than mutants with deletions of either phosphate acetyltransferase (pta) or pta–ptsG, due to the low accumulation of acetate and other byproducts, as well as high biomass production. The appropriate dissolved oxygen (DO) level, glucose feeding mode, and pH control strategy were applied to ʟ-tryptophan production using the BCTRPG mutant. The BCTRPG strain with optimized conditions resulted in a reduction in acetate accumulation (71.08% reduction to 0.72 g/L) and an increase in ʟ-tryptophan production (35.81% increase to 17.14 g/L) compared with the BCTRP strain in the original culture condition. Meanwhile, an analysis of the metabolic flux distribution indicated that the acetate synthesis flux decreased from 19.2% (original conditions) to 8.4% (optimized conditions), and the flux of tryptophan formation with the optimized conditions was 18.5%, which was 1.89 times higher than under the original conditions. This study provided the theoretical foundation and technical support for high-level industrialization production of ʟ-tryptophan.






Similar content being viewed by others
References
Åkesson M, Hagander P, Axelsson JP (2001) Avoiding acetate accumulation in Escherichia coli cultures using feedback control of glucose feeding. Biotechnol Bioeng 73(3):223–230
Báez-Viveros JL, Flores N, Juárez K, Castillo-España P, Bolivar F, Gosset G (2007) Metabolic transcription analysis of engineered Escherichia coli strains that overproduce ʟ-phenylalanine. Microb Cell Factories 6:30. doi:10.1186/1475-2859-6-30
Bongaerts J, Krämer M, Müller U, Raeven L, Wubbolts M (2001) Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab Eng 3(4):289–300
Castaño-Cerezo S, Pastor JM, Renilla S, Bernal V, Iborra JL, Cánovas M (2009) An insight into the role of phosphotransacetylase (pta) and the acetate/acetyl-CoA node in Escherichia coli. Microb Cell Factories 8:54. doi:10.1186/1475-2859-8-54
Chang DE, Shin S, Rhee JS, Pan JG (1999) Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme a flux for growth and survival. J Bacteriol 181(21):6656–6663
Cheng LK, Wang J, Xu QY, Xie XX, Zhang YJ, Zhao CG, Chen N (2012) Effect of feeding strategy on L-Tryptophan production by recombinant Escherichia coli. Ann Microbiol 62(4):1625–1634
Cheng LK, Wang J, Xu QY, Zhao CG, Shen ZQ, Xie XX, Chen N (2013) Strategy for pH control and pH feedback-controlled substrate feeding for high-level production of L-Tryptophan by Escherichia coli. World J Microbiol Biotechnol 29:883–890
Contiero J, Beatty CM, Kumari S, DeSanti CL, Strohl WR, Wolfe AJ (2000) Effects of mutations in acetate metabolism on high-cell-density growth of Escherichia coli. J Ind Microbiol Biotechnol 24(6):421–430
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645
De Anda R, Lara AR, Hernández V, Hernández-Montalvo V, Gosset G, Bolívar F, Ramírez OT (2006) Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metabol Eng 8(3):281–290
Dittrich CR, Vadali RV, Bennett GN, San KY (2005) Redistribution of metabolic fluxes in the central aerobic metabolic pathway of E. coli mutant strains with deletion of the ackA–pta and poxB pathways for the synthesis of isoamyl acetate. Biotechnol Prog 21:627–631
Dodge TC, Gerstner JM (2002) Optimization of the glucose feed rate profile for the production of tryptophan from recombinant E. coli. J Chem Technol Biotechnol 77:1238–1245
Eiteman MA, Altman E (2006) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24(11):530–536
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microb Cell Factories 4:14. doi:10.1186/1475-2859-4-14
Gu PF, Yang F, Kang JH, Wang Q, Qi QS (2012) One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of L-Tryptophan in Escherichia coli. Microb Cell Factories 11:30
Huang J, Shi JM, Liu Q, Xu QY, Xie XX, Wen TY, Chen N (2011) Effects of gene pta disruption on L-Tryptophan fermentation. Acta Microbiol Sina 51(4):480–487
Ikeda M (2006) Towards bacterial strains overproducing L-Tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol 69(6):615–626
Jiang PH, Shi M, Qian ZK, Li NJ, Huang WD (2000) Effect of F209S mutation of Escherichia coli AroG on resistance to phenylalanine feedback inhibition. Acta Biochim Biophys Sin 32(5):441–444
Lin H, Castro NM, Bennett GN, San KY (2006) Acetyl-CoA synthetase overexpression in Escherichia coli demonstrates more efficient acetate assimilation and lower acetate accumulation: a potential tool in metabolic engineering. Appl Microbiol Biotechnol 71:870–874
Liu Q, Cheng YS, Xie XX, Xu QY, Chen N (2012) Modification of tryptophan transport system and its impact on production of L-Tryptophan in Escherichia coli. Bioresour Technol 114:549–554
Lu J, Tang JL, Liu Y, Zhu XN, Zhang TC, Zhang XL (2012) Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol 93:2455–2462
Nakano K, Rischke M, Sato S, Maerkl H (1997) Influence of acetic acid on the growth of Escherichia coli K12 during high-cell-density cultivation in a dialysis reactor. Appl Microbiol Biotechnol 48(5):597–601
Noronha SB, Yeh HJC, Spande TF, Shiloach J (2000) Investigation of the TCA cycle and the glyoxylate shunt in Escherichia coli BL21 and JM109 using 13C-NMR/MS. Biotechnol Bioeng 68:316–327
Phue JN, Shiloach J (2004) Transcription levels of key metabolic genes are the cause for different glucose utilization pathways in E. coli B (BL21) and E. coli K (JM109). J Biotechnol 109:21–30
Phue JN, Shiloach J (2005) Impact of dissolved oxygen concentration on acetate accumulation and physiology of E. coli BL21, evaluating transcription levels of key genes at different dissolved oxygen conditions. Metab Eng 7(5–6):353–363
Picon A, Teixeira MJ, Postma PW (2005) Reducing the glucose uptake rate in Escherichia coli affects growth rate but not protein production. Biotechnol Bioeng 90:191–200
Postma PW, Lengeler JW, Jacobson GR (1993) Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57(3):543–594
Riesenberg D, Menzel K, Schulz V, Schumann K, Veith G, Zuber G, Knorre WA (1990) High cell density fermentation of recombinant Escherichia coli expressing human interferon alpha 1. Appl Microbiol Biotechnol 34(1):77–82
Schmid JW, Mauch K, Reuss M, Gilles ED, Kremling A (2004) Metabolic design based on a coupled gene expression—metabolic network model of tryptophan production in Escherichia coli. Metab Eng 6(4):364–377
Shen T, Liu Q, Xie XX, Xu QY, Chen N (2012) Improved production of tryptophan in genetically engineered Escherichia coli with TktA and PpsA overexpression. J Biomed Biotechnol. doi:10.1155/2012/605219
Sigala JC, Flores S, Flores N, Aguilar C, De Anda R, Gosset G, Bolívar F (2009) Acetate metabolism in Escherichia coli strains lacking phosphoenolpyruvate: carbohydrate phosphotransferase system; evidence of carbon recycling strategies and futile cycles. J Mol Microbiol Biotechnol 16:224–235
Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL (2002) pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J Bacteriol 184(15):4246–4258
Tang JL, Zhu XN, Lu J, Liu PP, Xu HG, Tan ZG, Zhang XL (2013) Recruiting alternative glucose utilization pathways for improving succinate production. Appl Microbiol Biotechnol 97:2513–2520
Wang J, Cheng LK, Wang J, Liu Q, Shen T, Chen N (2013) Genetic engineering of Escherichia coli to enhance production of L-Tryptophan. Appl Microbiol Biotechnol 97:7587–7596
Zhao CG, Cheng LK, Xu QY, Wang J, Shen ZQ, Chen N (2016) Improvement of the production of L-Tryptophan in Escherichia coli by application of a dissolved oxygen stage control strategy. Ann Microbiol 66:843–854
Zhu JF, Shimizu K (2005) Effect of a single-gene knockout on the metabolic regulation in Escherichia coli for d-lactate production under microaerobic condition. Metab Eng 7:104–115
Acknowledgments
This work was supported by the Doctoral Program Foundation of Shandong Binzhou Animal Science and Veterinary Medicine Academy (BS201402), Development of Science and Technology Plan Program of Binzhou (2015ZC0107), and Agricultural Science and Technology Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2017B02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the Doctoral Program Foundation of Shandong Binzhou Animal Science and Veterinary Medicine Academy (BS201402), Development of Science and Technology Plan Program of Binzhou (2015ZC0107), and Agricultural Science and Technology Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2017B02).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lu, N., Zhang, B., Cheng, L. et al. Gene modification of Escherichia coli and incorporation of process control to decrease acetate accumulation and increase ʟ-tryptophan production. Ann Microbiol 67, 567–576 (2017). https://doi.org/10.1007/s13213-017-1289-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-017-1289-8