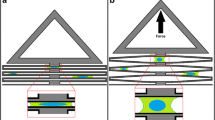
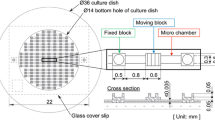
Abstract
Tensile stress is one of the most common mechanical stresses on the connective tissues of human organs. Cell stretching devices have been developed to study the effects of tensile stress on cells and tissues. In this study, we review how these devices function mechanically and apply them to biological research. To this end, we technically evaluate the four types of actuation processes used in cell stretching devices, including electric motor-driven and electromagnetic actuation, along with their pros and cons. For example, these cell stretching devices have shortcomings including large size, a complicated system, and generation of heat and shock, which hinder the real-time imaging of cells during stretching in high-resolution microscopes. We also describe the effects of tensile stress on cellular and tissue homeostasis. With this review, we seek to explore future directions for development of cell tensioning devices to understand mechanobiological responses to mechanical stress in vivo.





Similar content being viewed by others
References
Wang, J.H.C., Thampatty, B.P.: An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol 5, 1–16 (2006)
Ingber, D.E.: Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827 (2006)
Butcher, D.T., Alliston, T., Weaver, V.M.: A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009)
Sanderson, I.R.: The physicochemical environment of the neonatal intestine. Am. J. Clin. Nutr. 69, 1028s–1034s (1999)
Wang, L., et al.: Biomechanical studies on biomaterial degradation and co-cultured cells: mechanisms, potential applications, challenges and prospects. J. Mater. Chem. B 7, 7439–7459 (2019)
Huh, D., et al.: Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010)
Hoffman, B.D., Crocker, J.C.: Cell mechanics: dissecting the physical responses of cells to force. Annu. Rev. Biomed. Eng. 11, 259–288 (2009)
Happe, C.L., Engler, A.J.: Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ. Res. 118, 296–310 (2016)
Aragona, M., et al.: A mechanical checkpoint controls multicellular growth through YAP1/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013)
Labernadie, A., et al.: A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 (2017)
Cai, X., Wang, K.C., Meng, Z.: Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front. Cell Dev. Biol. 9, 673599 (2021)
Silver, F.H., Siperko, L.M.: Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix? Crit. Rev. Biomed. Eng. 31, 255–331 (2003)
DuFort, C.C., Paszek, M.J., Weaver, V.M.: Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell. Biol. 12, 308–319 (2011)
Kamble, H., et al.: Cell stretching devices as research tools: engineering and biological considerations. Lab Chip 16, 3193–3203 (2016)
Cui, Y., et al.: Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 6, 1–8 (2015)
Chiu, C.H., et al.: Osteogenesis and chondrogenesis of primary rabbit periosteal cells under non-uniform 2-axial tensile strain. BioChip J. 14, 438–446 (2020)
Trepat, X., et al.: Universal physical responses to stretch in the living cell. Nature 447, 592–595 (2007)
Kim, E.H., et al.: Effect of cyclic stretching on cell shape and division. BioChip J. 9, 306–312 (2015)
Samak, G., et al.: Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 306, 947–958 (2014)
Lee, C.-F., et al.: Cyclic stretch-induced stress fiber dynamics–dependence on strain rate, Rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 401, 344–349 (2010)
Gudipaty, S.A., et al.: Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543, 118–121 (2017)
Wyatt, T.P., et al.: Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc. Nat’l. Acad. Sci. U. S. A. 112, 5726–5731 (2015)
Chang, Y.J., et al.: Micropatterned stretching system for the investigation of mechanical tension on neural stem cells behavior. Nanomedicine 9, 345–355 (2013)
Ahn, J., et al.: A microfluidic stretch system upregulates resistance exercise-related pathway. BioChip J. (2022). https://doi.org/10.1007/s13206-022-00051-6
Kaunas, R., et al.: Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Nat’l. Acad. Sci. U. S. A. 102, 15895–15900 (2005)
Huang, L., et al.: A stretching device for high-resolution live-cell imaging. Ann. Biomed. Eng. 38, 1728–1740 (2010)
Harshad, K., et al.: An electromagnetic cell-stretching device for mechanotransduction studies of olfactory ensheathing cells. Biomed. Microdevices 18, 45 (2016)
Lim, H.Y., et al.: Development of wrinkled skin-on-a-chip (WSOC) by cyclic uniaxial stretching. J. Ind. Eng. Chem. 68, 238–245 (2018)
Iwadate, Y., et al.: Cyclic stretch of the substratum using a shape-memory alloy induces directional migration in Dictyostelium cells. Biotechniques 47, 757–767 (2009)
Wang, Q., et al.: A microscale mechanical stimulator for generating identical in-plane surface strains toward live cells on multiple loading sites. Sens. Actuators B Chem. 194, 484–491 (2014)
Gopalan, S.M., et al.: Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol. Bioeng. 81, 578–587 (2003)
Zhao, X.-H., et al.: Force activates smooth muscle α-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801–1809 (2007)
McGee, K.M., et al.: Nuclear transport of the serum response factor coactivator MRTF-A is downregulated at tensional homeostasis. EMBO rep. 12, 963–970 (2011)
Dong, J., et al.: Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007)
Eisenhoffer, G.T., et al.: Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012)
Coste, B., et al.: Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010)
Singhvi, R., et al.: Engineering cell shape and function. Science 264, 696–698 (1994)
Folkman, J., et al.: Role of cell shape in growth control. Nature 273, 345–349 (1978)
Matter, K., et al.: Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 17, 453–458 (2005)
Madara, J.L.: Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60, 143–159 (1998)
DeMeo, M.T., et al.: Intestinal permeation and gastrointestinal disease. J. Clin. Gastroenterol. 34, 385–396 (2002)
Bogoyevitch, M.A., et al.: Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. 70, 1061–1095 (2006)
Bogoyevitch, M.A., et al.: c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim. Biophys. Acta Proteins Proteom. 1804, 463–475 (2010)
Chaturvedi, L.S., et al.: Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J. Biol. Chem. 282, 14–28 (2007)
Li, W., et al.: Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G75–G87 (2001)
Thiery, J.P.: Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002)
Greenburg, G., et al.: Epithelia suspended in collagen gels can lose characteristics of migrating mesenchymal cells. J. Cell Biol. 95, 333–339 (1982)
Heise, R.L., et al.: Mechanical stretch induces epithelial-mesenchymal transition in alveolarepithelia via hyaluronan activation of innate immunity. J. Biol. Chem. 286, 17435–17444 (2011)
Acknowledgements
This research was equally supported by the Fostering Global Talents for Innovative Growth Program (P0008746) supervised by the Korean Institute for Advancement of Technology (KIAT) and by the Technology Innovation Program (Industrial Strategic Technology Development Program-Development of disease models based on 3D microenvironmental platforms mimicking multiple organs and evaluation of drug efficacy) (20008413) funded by the MOTIE (Ministry of Trade, Industry, and Energy) in Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Kim, S., Uddin, S. et al. Microfabricated Stretching Devices for Studying the Effects of Tensile Stress on Cells and Tissues. BioChip J 16, 366–375 (2022). https://doi.org/10.1007/s13206-022-00073-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-022-00073-0