Abstract
Ultrafine cobalt–nickel bimetallic phosphides were prepared by mild hydrothermal method and characterized by techniques, such as XRD, EDS, and TEM. The results showed that the as-prepared products were well crystallized and particle sizes ranged from 10 to 20 nm. With the increasing amount of Ni2+ in the suspensions, the crystallinity of obtained products was improved gradually and the crystallite sizes increased accordingly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metal phosphide compounds have great application prospects in the catalytic agent (Brock and Senevirathne 2008; Rodriguez et al. 2003) due to their excellent antisulfur-poisoning property and superior catalytic activity. They are the promising candidates in industrial application and have attracted the attention of many researchers. Considering this, many scientists have done plentiful research work in this field. They have investigated a series of synthesis methods, such as chemical combination by elementary substances directly (Rundqvist 1966), replacement reaction by solidity (Fjellvag et al. 1984) chemical reaction between metal halide lamp and phosphate (Rowley and Parkin 1993), and decomposition of organic compound of metal (Gingerich 1964). However, these procedures typically suffer from relatively high reaction temperature and long annealing periods.
Aitken et al. (2005) have firstly reported solvothermal syntheses of Cu3P. Thereafter, our group has synthesized Ni2P, Ni12P5, and Co2P with hydrothermal method using red phosphorus as raw material (Liu et al. 2010; Huang et al. 2010, 2011). Until now, few related studies have paid any attention to the above-mentioned synthesis process. In this study, we synthesized ultrafine cobalt–nickel bimetallic phosphide powders with red phosphor, which was a benign and substantial phosphor source to synthesize transition metal phosphide. In addition to the advantageous raw material, the hydrothermal experiments were conducted under a relative low temperature (200°C) for only 10 h.
Experiment
Preparation of the starting suspension
Starting materials were analytical reagents: NiCl2·6H2O (≥99.0%), CoSO4·7H2O (≥99.5%), and red phosphor (Fig. 1). The hydrothermal synthesis process was carried out as follows. Firstly, a desired amount of NiCl2·6H2O and CoSO4·7H2O were added to distilled water to get a mixed aqueous solution of Ni2+ and Co2+. Then red phosphor was added to the solution under vigorous stirring to get the starting suspension. Concentrations of the suspensions and hydrothermal conditions of the experiments are presented in Table 1. In this work, the molar ratio of Co2+ and P remained 1:5, and the amount of Ni2+ was adjusted according to Table 1.
Preparation of the samples
The prepared suspension was poured into a Teflon-lined autoclave with 0.8 filling factor, sealed, and hydrothermally treated at 200°C for 10 h. After the autoclave cooled to room temperature, the black products were collected and washed with plenty of distilled water. They were then dried at 50°C for 5 h in the air.
Characterization
Phase constitution, chemical composition, and morphology were characterized by X-ray powder diffraction (XRD; Model D/max Rigaku Co., Japan) with Cu Kα radiation (40 kV, 150 mA), energy dispersive X-ray spectroscopy (Oxford Instruments’ INCA EDS system) and transmission electron microscopy (TEM; Model JEM-1200EX, JEOL Co, Japan), respectively.
Results and discussion
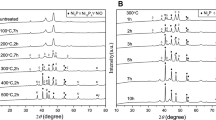
Figure 2 presents the XRD patterns of samples from different ratios of Ni/Co/P prepared at 200°C for 10 h. It showed that products of Groups 1–5 (especially for Groups 2 and 3) were well crystallized. All the reflection peaks had a good agreement with the reported results so far (Burns et al. 2008; Aal et al. 2008). Along with the decreasing amount of initial Ni2+, peaks of Groups 4 and 5 became relatively wider and smaller than those of Groups 1–3. When the ratio of Ni/Co/P was 1:25:125 (Group 6), the main peaks were hardly observed which should be attributed to small crystallite sizes. Based on XRD results, the mean crystal size of the obtained products was about 10–20 nm estimated by Scherrer equation through half width of the main peak in the 40° region. It was obvious that with the increasing amount of Ni2+ in the suspensions, the crystallinity of obtained products was improved gradually and the crystallite sizes increased accordingly.
The EDS spectra of Groups 2 and 3 in Fig. 3 show the presence in the products of Ni, Co and P, and there are no other impurity peaks in the spectra. The Ni/Co/P ratios were 50:4:20 and 44:4:19, respectively. The EDS results confirmed the chemical composition of the as-prepared products and agreed well with the XRD results in Fig. 2.
Transmission electron microscopy and selected area electron diffraction (SAED) images of the obtained products from Groups 2 and 3 are shown in Fig. 4. The powders were composed of irregular aggregates forming from nanosize crystallites (10–20 nm estimated by Scherrer equation). SAED patterns recorded on particle in the TEM image confirmed that our products were made up of nanocrystalline compounds that showed characteristic diffuse and faint electron diffraction rings. And it also indicated that the as-prepared aggregates were polycrystal particles (Revaprasadu et al. 1999; Yang et al. 1998).
Conclusion
Cobalt–nickel bimetallic phosphide nanoparticles were successfully synthesized by the hydrothermal method at 200°C for 10 h. The sizes of as-prepared products were 10–20 nm. With the increasing amount of Ni2+ in the suspensions, the crystallinity of obtained products was improved gradually.
References
Aal A, Shaaban A, Abdel Hamid Z (2008) Nanocrystalline soft ferromagnetic Ni–Co–P thin film on Al alloy by low temperature electroless deposition. Appl Surf Sci 254:1966–1971
Aitken JA, Ganzha-Hazen V, Brock SL (2005) Solvothermal syntheses of Cu3P via reactions of amorphous red phosphorus with a variety of copper sources. J Solid State Chem 178:970–975
Brock SL, Senevirathne K (2008) Recent developments in synthetic approaches to transition metal phosphide nanoparticles for magnetic and catalytic applications. J Solid State Chem 181:1552–1559
Burns AW, Gaudette AF, Bussell ME (2008) Hydrodesulphurization properties of cobalt–nickel phosphide catalysts: Ni-rich materials are highly active. J Catal 260:262–269
Fjellvag H, Kjekshus A, Andresen AF (1984) Magnetic and structural properties of transition metal substituted MnP. III. Mn1-tFetP (0.00 ≤ t ≤ 0.30). Acta Chem Scand A 38:709–718
Gingerich KA (1964) Vaporization behavior and phosphorus decomposition pressures of tungsten monophosphide. J Phys Chem 68:768–772
Huang X, Liu Z, Dai J, Zhu Z (2010) A hydrothermal method for the synthesis of phosphides. CN Patent 101659403B
Huang H, Huang X, Zhu Z, Dai J (2011) Hydrothermal synthesis of cobalt phosphide nanoparticles. Ceram Int. doi:10.1016/j.ceramint.2011.09.001
Liu Z, Huang X, Zhu Z, Dai J (2010) A simple mild hydrothermal route for the synthesis of nickel phosphide powders. Ceram Int 36:1155–1158
Revaprasadu N, Azad Malik M, Carstens J, O’Brien P (1999) Novel single-molecule precursor routes for the direct synthesis of InS and InSe quantum dots. J Mater Chem 9:2885–2888
Rodriguez JA, Kim J-Y, Hanson JC (2003) Physical and chemical properties of MoP, Ni2P, and MoNiP. J Phys Chem B 107:6276–6285
Rowley AT, Parkin IP (1993) Convenient synthesis of lanthanide and mixed lanthanide phosphides by solid-state routes involving sodium phosphide. J Mater Chem 3:689–692
Rundqvist S (1966) New metal-rich phosphides of niobium, tantalum, and tungsten. Nature 211:847–848
Yang P, Zhao D, Margolese DI, Chmelka BF, Stucky GD (1998) Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 396:152–155
Acknowledgments
Financial support from the Ocean University of China through Young Teachers’ Scientific Research Special Fund Projects (No. 201113038) is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wang, B., Huang, X., Zhu, Z. et al. Hydrothermal synthesis of cobalt–nickel bimetallic phosphides. Appl Nanosci 2, 481–485 (2012). https://doi.org/10.1007/s13204-012-0062-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0062-3