Abstract
Glycerol carbonylation with urea is a very feasible option to produce glycerol carbonate with a net result of CO2 fixation through urea synthesis. The prerequisite of an efficient catalyst for this reaction is to possess both acid and basic sites together. Several acidic supports were screened for ZnO catalyst in this work and Zn/MCM-41 was found to exhibit the best activity and almost complete selectivity to glycerol carbonate (GC). Although, non-catalytic glycerol carbonylation resulted in GC formation but glycerol conversion achieved was twice with Zn/MCM-41 as a catalyst. Further to that increase in Zn loading from 2 to 5% resulted in increase in glycerol conversion from 63 to 82%. The prepared catalysts were characterized by XRD, NH3 and CO2-TPD and effects of reaction parameters such as catalyst loading, glycerol to urea mole ratio and temperature on glycerol conversion and GC selectivity in batch mode of operation were also studied. Time on stream activity of 5% Zn/MCM-41 catalyst for continuous carbonylation of glycerol was also studied for ~100 h with an average conversion of ~55% and complete selectivity to GC. This indicated five times lower productivity of GC per h due to lower residence time than that in a batch operation as compared to that of a continuous operation. Activation energy estimated from the Arrhenius plot was found to be 39.82 kJ mol−1 suggesting that the reaction is kinetically controlled. A reaction pathway mediated by acid and basic sites of the Zn/MCM-41 catalyst is also proposed.
Similar content being viewed by others
Introduction
Biodiesel produced by transesterification of triglycerides has created a practical option at least partially, for fossil fuel [1]. In recent years, increased biodiesel production has also led to a large scale availability of glycerol which necessitates its valorization by exploring new avenues [2]. Arising from the need of utilization of bioglycerol, one of the attempts involves the synthesis of glycerol carbonate which is a high value monomer for polycarbonate synthesis as well as a polar solvent having an excellent biodegradability, low toxicity and miscibility with water [3–5]. Glycerol carbonate (GC) is also used as an intermediate in the synthesis of pharmaceuticals, fine chemicals and agricultural chemicals [3–5].
GC synthesis has been reported primarily by catalytic carbonylation of glycerol with CO/O2 or phosgene. The oxycarbonylation of diols also has been reported using Pd/Mn redox catalyst system [6]. Nevertheless, use of CO or phosgene in such processes poses serious environmental and handling drawbacks due to their hazardous nature [7–10]. Direct carbonylation using CO2 although a preferred route for making GC, it is thermodynamically unfavorable offering very low yield (<25%) of GC [11, 12]. Attempts made for CO2 activation involved very high pressure (>5 MPa) and temperature (>180 °C) conditions with <50% glycerol conversion and trace formation of GC [13]. In another variation, cycloaddition of CO2 with glycidol resulted into higher GC yield of >92%. However the starting material glycidol obtained from GC is costlier than GC, making this process uneconomical for industrial exploitation [11]. Glycerol transesterification with alkyl carbonates can be one of the viable options for GC in which complete glycerol conversion with >75% GC selectivity has been reported [12]. However, making of alkyl carbonates in turn proceeds through phosgene or energy intensive epoxide routes. GC can also be produced by direct carbonation of glycerol with ethylene carbonate under supercritical CO2 using zeolite as a catalyst [14]. However, there is no evidence that a direct CO2 insertion occurred and glycerol carbonate was only produced when another organic carbonate was added as a reactant. The most widely studied route of GC synthesis is the transesterification of glycerol with dimethyl carbonate (DMC) [15, 16], ethylene carbonate (EC) [17, 18] and propylene carbonate (PC) [19]. However, these cyclic carbonates used for transesterification are typically synthesized either from phosgene utilization or energy intensive route involving epoxide. Use of EC leads to formation of ethylene glycol that involves tedious work-up procedure. Although DMC was exploited for transesterification with glycerol using basic catalysts like K2CO3, CaO and Ca/La mixed oxide but the recyclability of these catalysts was not established [20, 21]. Compared to the above-mentioned routes for GC, carbonylation of glycerol with urea proves to be greener, cheaper and atom economic for the synthesis of GC. Urea produced by reaction of CO2 and ammonia with a worldwide capacity of 108 tpa is the well established example of chemical utilization of CO2. It follows that glycerol carbonylation via urea route establishes the indirect utilization of CO2. Moreover, NH3 evolved in this carbonylation process becomes available again for urea production (Scheme 1). Among the several catalytic systems reported for this reaction, homogeneous catalysts include the salts of Mn, Zn and Zr [13, 22–25], while heterogeneous systems include Zn-hydrotalcite [25], Co3O4/ZnO [26], solid base like La2O3 etc. [27]. It is exceptional that Hammond et al. reported noble metal like Au supported on MgO but with glycerol conversion of 88% and a much lower selectivity of 56% to GC [28]. Recently, Cs-exchanged heteropolyacid functionalized with Sn catalysts were reported giving glycerol conversion of 91% however, GC selectivity was hampered (83%) due to the formation of undesired byproducts such as GD and glycerol urethane [29]. These systems reported so far have some other common drawbacks of higher catalyst to substrate ratio, use of high vacuum for NH3 removal thus making the process cost intensive in terms of higher energy and catalyst consumption.
Recently, we have reported screening study of various MCM-41 supported Cu, Ni and Zn oxide catalysts for glycerol carbonylation with urea to give GC [30]. In continuation, we studied in detail the role of different supports and loading of Zn in glycerol-urea reaction to produce GC. Effect of reaction parameters such as temperature, catalyst loading and glycerol to urea ratio was also performed to arrive at optimized reaction conditions. In addition, the performance comparison in batch and continuous operations showed that much higher conversion was obtained in a batch process due to higher residence time in the later.
Experimental details
Catalysts preparation
The support MCM-41 was first prepared by hydrothermal method reported in the literature [30]. In a typical preparation, 2.67 g of sodium hydroxide was dissolved in 146 g of distilled water and stirred for 5 min. To that solution, 5.96 g of cetyltrimethylammonium bromide (CTAB, Aldrich Chemicals, Bangalore, India) was added and the mixture was stirred for 15 min. To this, 14 g of tetraethyl orthosilicate (TEOS) was added drop wise and pH of the gel was maintained at 9–10 using dilute H2SO4 solution. The resultant gel was stirred for further 5 h at room temperature. It was then transferred into a Teflon-lined stainless steel autoclave and heated to 373 K for 48 h. The solid product was filtered, washed with distilled water, dried at 353 K and finally calcined at 813 K for 6 h.
5% Zn catalysts on different supports like MCM-41, SBA-15, ZrO2, SiO2, Al2O3 and sulphated ZrO2 (s-ZrO2) were synthesized by wet impregnation method. In a typical procedure, 0.33 g of zinc acetate dihydrate (Zn (O2CCH3)2(H2O)2) was dissolved in 10 mL of water and was stirred for 5 min, followed by the slow addition of the respective support to the solution. The solution was kept under stirring for 6 h at room temperature in nitrogen atmosphere. Then the solvent was evaporated on a rotavap and the catalysts were calcined at 813 K for 3 h. The prepared catalysts were nominated as Zn/MCM-41, Zn/SBA-15, Zn/ZrO2, Zn/SiO2, Zn/Al2O3 and Zn/sulfated ZrO2, respectively.
Characterization
The crystallinity and phase purity of all the prepared catalysts were analyzed by X-ray diffraction (XRD) patterns on a P Analytical PXRD system (Model X-Pert PRO-1712), using Ni filtered Cu Kα radiation (λ = 0.154 nm) as an X-ray source (current intensity, 30 mA; voltage, 40 kV) and an X-accelerator detector. The samples were scanned in a 2θ range of 0o–10o and 10o–90o. The species present in the catalysts were identified by their characteristic 2θ values of the relevant crystalline phases. The software program X-Pert High Score Plus was employed to subtract the contribution of the Cu Kα 2 line prior to data analysis. BET surface areas and NH3, CO2-TPD measurements were carried out by the adsorption/desorption of nitrogen (77 K), NH3, CO2, respectively, using Micromeritics Chemisorb 2720 instrument. To evaluate acidity and basicity of the catalysts, ammonia and carbon dioxide TPD measurements were carried out by: (1) pre-treating the samples from room temperature to 200 °C under helium flow rate of 25 mL min−1, (2) adsorption of ammonia/carbon dioxide at 40 °C and (3) desorption of ammonia/carbon dioxide with a heating rate of 10 °C min−1 starting from the adsorption temperature to 700 °C. The particle size, morphology, and elemental composition were studied using transmission electron microscope (HR-TEM), model JEOL 1200 EX. For this purpose, the sample was prepared by sonicating a small amount of the solid sample in ethanol for half an hour. A drop of the prepared suspension was deposited on a Cu grid coated with carbon layer and the grid was dried at room temperature before the analysis.
Activity testing
Batch operation
The catalytic reaction of glycerol and urea was carried out in a 50 mL three neck round bottom flask, equipped with a condenser. In a typical reaction, respective catalyst (0.23 g), glycerol (50 mmol), and urea (50 mmol) were charged into the reactor. The reaction mixture was heated in a silicon oil bath at 140 °C in the absence of any solvent under nitrogen flow of about 10 mL h−1. After completion of the reaction, 10 mL of methanol (MeOH) was added in the reaction crude followed by filtration to remove the catalyst. Repeated washings with MeOH were given to the solid catalyst. Liquid samples were withdrawn from the reactor and analyzed by gas chromatography to monitor the progress of the reaction. For this purpose, the gas chromatograph, Shimatzu 2025 equipped with HP-FFAP column (30 m length × 0.53 mm ID × 1 μm film thickness) and a flame ionization detector was used.
Continuous operation
Continuous carbonylation of glycerol was carried out in a bench-scale, fixed bed reactor supplied by M/s Geomechanique, France. The powdered catalyst was pelletized in the form of pellets of 1.3 cm diameter and cut into four pieces each having 0.65 cm diameter (Fig. 1). 3 g of the pelletized catalyst was charged to the reactor. Reaction conditions used for the continuous operation are given in Table 1. The section of 17.4 cm above and 13 cm below the catalyst bed was packed with carborundum as an inert packing, thus providing the length of catalyst bed of 8.1 cm. Before starting the actual experiment, a flow of N2 (20 mL h−1) was maintained through the reactor. The liquid feed was “switched on” after attaining the desired temperature. The reactor feed flow was maintained for 1 h to obtain the constant liquid flow rate. Liquid samples were withdrawn at regular intervals of time. Liquid samples were analyzed by Shimatzu 2025 GC equipped with HP-FFAP column (30 m length × 0.53 mm ID × 1 μm film thickness) and a flame ionization detector. Following this procedure, the experiments were carried out at different inlet conditions. Steady-state performance of the reactor was observed by analysis of the reactant and products in the exit stream.
Results and discussion
Catalyst characterization
Glycerol carbonylation with urea requires both acid and base sites; hence, the primary aim of this work was to understand the role of support and Zn loading in this reaction. For this purpose, a systematic characterization of the catalysts was carried out as discussed below. Table 2 shows the surface areas of different supports for a constant loading of Zn (5%). As seen from Table 2, Zn supported on MCM-41 catalyst showed the maximum surface area of 692 m2 g−1 which varied with the supports in the descending order of MCM-41 > SBA-15 > SiO2 > γ-Al2O3 > ZrO2 > s-ZrO2 in accordance with the nature of the support material (discussed later). MCM-41 having the maximum surface area was used for further study on Zn loading variation in the range of 2–10%. Figure 2 shows Zn loading vs. surface area along with GC yield profiles. As expected, surface area decreased from 692 to 498 m2 g−1 with increase in Zn loading from 2 to 10%. Although the trend is not linear, it indicates the dispersion of Zn within the mesoporous structure of silicalite MCM-41 and simultaneously affecting the uniform ordering of the mesoporous structure as can be seen from the XRD analysis. The increased dispersion of Zn with increase in loading has directly reflected in the enhancement of GC yield from 57 to 83%. This suggests that the catalytic activity cannot be simply correlated to the surface area of the catalysts. Further interpretation of the activity results was deduced from various surface acid–base properties and structural ordering of the support material.
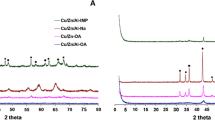
Figure 3A displays small-angle XRD patterns of MCM-41 and SBA-15 supported Zn catalysts which showed good ordering of 2D-hexagonal structure indexed to (100), (110) and (200) reflections [31, 32]. This suggests the retention of hexagonal mesoporous ordering of the MCM-41 and SBA-15 even after Zn loading. Wide angle XRD patterns (Fig. 3B) of 5% Zn on MCM-41, SBA-15 and SiO2 show the amorphous nature of the samples while a single broad peak at 2θ = 23.1o (411) could be attributed to orthorhombic silica. There was no any reflection corresponding to ZnO phase, suggesting the well dispersed nature of ZnO particles on the support and/or inside the framework of mesoporous supports like MCM-41 and SBA-15 which is in accordance with the previous reports [33, 34]. In case of alumina support (Fig. 3B), peaks observed at 2θ = 37.2o (110), 42.0o (006), 45.3o (202) and 67.1o (214) were due to Al2O3 (JCPDF No. 81-1667) with a small peak at 2θ = 31.7o (100) of ZnO phase. The crystalline nature of 5% Zn/ZrO2 and s-ZrO2 samples was evident by the sharp peaks (Fig. 3B) at 2θ = 24.4o (110), 28.2o (ī11), 41.1o (102), 49.3o (022), 50.6o (ī22) and 59.9o (131) which were attributed to ZrO2 (JCPDF No. 83-0944). The sharp peaks observed at 2θ = 31.7o (100), 34.4o (002), 36.2o (101) and 66.4o (200) could be assigned to ZnO phase (JCPDF No. 80-0075). This confirmed that the formation of crystalline and/or large size ZnO particles was possible on ZrO2 and s-ZrO2 supports compared to MCM-41, SBA-15, SiO2 and Al2O3 supports. Nature of the support obviously influenced the surface areas of the respective catalysts, as discussed above.
As 5% Zn/MCM-41 showed the highest activity, effect of Zn loading on MCM-41 on structure and activity was also investigated. The low and wide angle XRD patterns of the parent MCM-41 and samples with Zn loading varying in the range of 2-10% are shown in Fig. 4A, B, respectively. Typical small-angle XRD of MCM-41 showed the reflections of (100), (110) and (200) planes corresponding to the ordered hexagonal mesoporous structure. In all the Zn-loaded samples, the presence of main (100) peak confirmed the unchanged hexagonal frame structure. However, loading of Zn varied from 2 to 10%, caused shifting of the main peak (100) to higher 2θ values with a peak broadening. The other two peaks (110) and (200) with low intensity confirmed the presence of ZnO within the pores of MCM-41 [35]. In case of highest Zn loading of 10%, the reflection of (100) plane weakened considerably while reflections of peaks due to (110) and (200) planes almost completely vanished. Thus, higher Zn loading dramatically influenced the structural ordering of MCM-41 [36]. The wide angle XRD patterns for various Zn loadings (Fig. 4B) did not show any peak corresponding to ZnO confirming excellent dispersion of ZnO inside the pores of MCM-41. The presence of Zn (ZnO) inside the framework of the MCM-41 was also confirmed by TEM analysis coupled with EDX (Fig. 5A, B).
Activation of glycerol and urea requires both acid and base sites, respectively, to form glycerol carbonate. Hence, the catalysts prepared in this work were characterized for their acid and base strength by NH3 and CO2-TPD, respectively. In NH3-TPD plots of Zn catalysts with different supports (Fig. 6), the peaks below 200 °C correspond to physisorbed NH3 whereas the peaks in the temperature range of 200–400 °C represent moderate acidity while, the peaks in the range of 400–700 °C represent the strong acidity. Only in case of ZrO2 and s-ZrO2 supported Zn catalysts, a sharp peak observed in the range of 650–700 °C clearly suggests strong acidic strength of both the catalysts [35]. Among all the prepared catalysts, SiO2 supported catalyst showed a broad peak below 200 °C with the lowest strength of total acidity of 0.1547 mmol g−1 in terms of NH3 desorbed. In case of Al2O3 supported catalyst, a broad distribution of acidity all over the temperature range of 100–700 °C was observed which clearly suggests the presence of weak, moderate as well as strong acidic sites [37]. MCM-41 supported Zn catalyst showed only a single broad peak below 200 °C indicating the presence of weak acid sites.
Table 3 shows comparative data of total acidity, basicity and acid/base ratio of the various loadings of Zn on MCM-41. Acidity of the catalysts initially increased with increasing Zn content up to 5% beyond which it remained almost constant at about 0.52 mmol of NH3 g−1. The increased acidity after Zn incorporation into the silica framework was due to the formation of Zn2+ Lewis acid sites in the silica framework as well as the BrØnsted acidity due to surface silanol groups. Similar observation was also described by Silvestre-Albero et al. for Zn supported MCM-41 catalyst [38]. Similar to the acidity trend, basicity generated due to the oxygen anion of the ZnO was less in case of 2% Zn loading which then increased for 5% loading and afterwards, it remained constant. The acidity/basicity ratio was found to be constant (~0.8) for the range of 5–10% Zn loading on MCM-41. It was observed that glycerol conversion increased substantially with increase in Zn loading irrespective of the acid/base ratio.
Carbonylation of glycerol with urea in a batch operation
Various supports were screened for constant loading of 5% Zn for the carbonylation of glycerol with urea and the results are shown in Table 4. Zn/MCM-41 showed highest conversion of 78% with maximum GC selectivity of 98% while, Zn/SiO2 showed the lowest conversion of 20% but 97% selectivity to GC. In case of Al2O3, ZrO2 and s-ZrO2 supported Zn catalysts, glycerol conversion was about 60% but the GC selectivity decreased substantially to 67%. Since, both Al2O3 and ZrO2 were having strong acidic sites (Fig. 6), formation of other side products such as urethanes was possible, reducing the GC selectivity [39]. It was clear from these results that the support such as MCM-41 having highest surface area was responsible for the maximum dispersion of active ZnO phase although having weak acidity, led to the highest activity. However, the selectivity to GC was strongly dependant on the appropriate acidity of the catalyst. Among the screened catalysts, as the Zn/MCM-41 showed the maximum glycerol conversion and GC selectivity further studies of process optimization and catalysts stability were performed using Zn/MCM-41 catalyst and these results are discussed below.
Effect of metal loading
Effect of active metal loadings on MCM-41 support in the range of 2–10% on conversion and selectivity of GC is shown in Table 5. Surprisingly, glycerol carbonylation with urea was also observed in absence of catalyst as well as with bare MCM-41 (entries 1 and 2, Table 5). The role of Zn as a catalyst was evident from the enhanced glycerol conversion and GC selectivity (entries 3–5, Table 5). The conversion of glycerol increased linearly from 63 to 82% which might be because of increase in both Lewis acid and base strength (Table 3) with increase in active metal loading from 2 to 5% while, further increase in metal loading to 10% did not affect the conversion significantly. GC selectivity observed was 92% for minimum Zn loading of 2% while, almost complete (~98%) selectivity to GC was achieved for Zn loading of 5 and 10% (entries 4–6, Table 5).
Effect of catalyst loading
The effect of loading of 10% Zn/MCM-41 catalyst was also studied in the range of 0.06–0.23 g, on activity and GC selectivity. As seen from Fig. 7, the conversion of glycerol increased from 70 to 85% while, selectivity to GC reached almost completion (from 97 to 98%), with about a fourfold increase in catalyst loading (0.06–0.23 g). This is obvious as increase in catalyst loading caused an increase in the acidic and basic sites that facilitated the carbonylation of glycerol with urea to give GC.
Effect of mol ratio
The effect of the mol ratio of glycerol to urea in the range of 1:0.5–1:2 on conversion and selectivity to GC was studied by varying the initial concentration of urea at a constant glycerol and catalyst concentration for 10% Zn/MCM-41 catalyst and the results are presented in Table 5. The conversion of glycerol was much lower (44%) for the highest mol ratio of glycerol to urea (entry 1, Table 6) obviously as urea was the limiting reactant. At higher urea concentration, glycerol conversion increased up to 92% (entries 2 and 3, Table 6); however, GC selectivity dropped down considerably from 98 to 58% (entry 3, Table 6). This is because at higher urea concentration, further carbonylation of GC with urea is possible forming urethanes [38]. At 1:1 mol ratio, highest selectivity of 98% to GC was achieved with 84% conversion, suggesting that 1:1 was an optimum ratio of glycerol to urea to give the highest activity and selectivity to GC.
Effect of the temperature
With the glycerol to urea ratio of 1:1, study of effect of temperature was done by varying the temperature in a range of 120–160 °C. As shown in Fig. 8, glycerol conversion linearly increased from 56 to 95% with increase in temperature from 120 to 160 °C. However, at higher reaction temperature of 160 °C, slight decrease in GC selectivity was observed with simultaneous increase in byproduct selectivity. Since, the maximum selectivity of 98% to GC was achieved at 140 °C, it was considered as the optimum reaction temperature.
Effect of the N2 flow rate
The effect of N2 flow rate was also studied by its variation as 10, 20 and 40 mL/h at optimum reaction temperature of 140 °C (Table 7). It was observed that both glycerol conversion and GC selectivity increased from 60 to 84% and from 86 to 98%, respectively, with increase in N2 flow from 10 to 20 mL/h. However, further rise in N2 flow of 40 mL/h showed only marginal increase in glycerol conversion of 87% while, GC selectivity remaining constant at 98%. Therefore, the optimum N2 flow was considered to be 20 mL/h.
Continuous operation
From the process point of view, it was thought appropriate to study the glycerol-urea carbonylation in a continuous mode of operation. From the activity study in a batch operation, it was observed that 10% Zn loading showed only marginal increase in conversion with the same selectivity to GC as that of 5% Zn/MCM-41catalyst (Table 5). Hence, further study was done with 5% Zn/MCM-41 catalyst in a continuous mode of operation.
Initially, time on stream activity of 5% Zn/MCM-41 catalyst was evaluated for continuous glycerol carbonylation in presence of 2-propanol (IPA) as a solvent. Figure 9 shows a consistent performance for 24 h of reaction time at 140 °C temperature and 20 mL h−1 N2 flow with an average glycerol conversion of ~25% and GC selectivity in the range of 70–75%. However, unlike the product distribution observed in the batch operation, selectivity to GC was restricted to only 75% with ~10% selective formation of GD and ~15% selectivity to other byproducts (Fig. 9). The possible byproducts included isopropyl carbonate and di-isopropyl carbonate obtained by transesterification of IPA with urea.
Figure 10 displays the time on stream (TOS) activity of the 5% Zn/MCM-41 catalyst in an aqueous medium for the carbonylation of glycerol to glycerol carbonate at 140 °C temperature, and 20 mL h−1 N2 flow. Interestingly, in contrast to IPA run, here the selectivity to GC enhanced to ~91% with ~9% formation of GD and other byproducts. The run started with the higher glycerol conversion of 35% which then stabilized to 25%. As the change from organic solvent (IPA) to aqueous, drastically enhanced GC selectivity from 75 to 91% further studies of process optimization were done in an aqueous medium.
Conversion and selectivity versus time profile of aqueous glycerol carbonylation in a continuous fixed bed reactor (reaction conditions: GLY-to-urea mol ratio = 1/1, catalyst wt. 5% Zn/MCM-41 = 3 g, 100 mL water solvent, 140 °C, 50 h, LHSV = 0.34/h, N2 flow = 20 mL/h to remove NH3 formed during reaction)
Optimization of operating conditions
Glycerol conversion and GC selectivity are greatly influenced by process parameters such as residence time, reaction temperature and glycerol concentration; hence, the effect of these parameters on the catalyst performance was carried out systematically and the results of which are discussed below.
The effect of liquid flow rate in terms of contact time on glycerol conversion is displayed in Fig. 11. It was observed that as the contact time increased from 0.2 to 0.3 h, a sharp rise in the glycerol conversion from 20 to 45% was observed with almost complete selectivity to GC. Further increase in contact time from 0.3 to 0.6 h caused linear increase in activity in terms of conversion from 45 to 55% with complete selectivity to GC. This follows that increase in residence time directly enhanced the number of possible collisions of the reactant molecules on the surface of the catalyst for lower liquid feed rate. Thus, the highest productivity of GC was achieved at 10 mL h−1 flow rate of aqueous glycerol. The effect of liquid flow rate on the global rate of glycerol carbonylation was also studied (Fig. 12) keeping all the other parameters constant. The linear decline in global rate from 0.7 × 10−8 to 0.09 × 10−8 k mol m−3 s−1 was observed with increase in liquid flow rate from 5 mL to 15 mL h−1. This may be due to the fact that in the lower range of liquid flow rate of 5–7 mL h−1, catalyst particles get partially wetted due to capillary action which is responsible for the direct interaction of liquid phase and solid surface of the catalyst, ultimately resulting in higher rate of carbonylation. In contrast to this, at higher liquid flow rates of 7–15 mL h−1, channelizing would hinder the access of liquid reactants to the catalyst sites resulting in the linear decrease in the global rate of carbonylation from 0.4 × 10−8 to 0.09 × 10−8 k mol m−3 s−1.
Figure 13 exhibits the effect of temperature on the glycerol conversion and GC selectivity in the carbonylation of glycerol with urea. With increase in temperature from 140 to 200 °C, a gradual increase in the glycerol conversion was observed along with simultaneous increase in GC selectivity from 91 to 100%. The rise in temperature from 140 to 170 °C resulted in marginal increase in glycerol conversion from 25 to 30%. However, further increase in temperature up to 200 °C, resulted in considerable increase in glycerol conversion from 30 to 45% with complete selectivity to GC without formation of any byproduct. Therefore, the optimum reaction temperature for the glycerol carbonylation was found to be 200 °C. Activation energy estimated from the Arrhenius plot was found to be 39.82 kJ mol−1, suggesting glycerol carbonylation reaction was kinetically controlled, under the present set of reaction conditions (Fig. 14).
Effect of glycerol loading on the catalytic activity for the glycerol carbonylation is shown in Fig. 15. Increase in glycerol loading from 26 to 36% caused twofold decrease in glycerol conversion was observed, without affecting the GC selectivity of 100%. This indicates that increased glycerol loading resulted in increased viscosity of the solution due to which substrate get strongly adsorbed on the active sites of the catalyst leading to the deactivation of catalytic activity.
Figure 16 shows the time on stream activity of 5% Zn/MCM-41 catalyst of 100 h at 200 °C, 10 mL h−1 feed flow and 20 mL h−1 N2 flow. Glycerol conversion was 50% within first 2 h after which it decreased slightly to 45% and then remained constant up to ~97 h of reaction time whereas, the GC selectivity was not affected (100%). As we compared the GC productivity in batch and continuous operation, the GC productivity in batch was five times (6.9 h−1) higher than that of continuous operation (1.38 h−1). This was due to higher residence time in a batch operation.
To confirm the glycerol conversion determined by gas chromatography of liquid samples, the quantification of NH3 released during the reaction was also carried out for few experiments. For this purpose, NH3 evolved from the reaction was dissolved in water at the reactor outlet using N2 as a carrier gas. The total amount of ammonia released was analyzed by volumetric analysis of both the reactor outlet gas sample and liquid reaction crude against strong 0.5 M HCl. The total amount of NH3 released was found to be 2.71 g which was in good agreement with the theoretically obtained value for 45% of glycerol conversion.
A possible reaction mechanism for carbonylation of glycerol with urea over Zn/MCM-41 catalyst
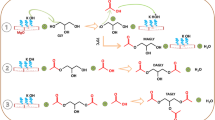
It is important to note that the present reaction of glycerol with urea to form GC proceeds without a catalyst under standard reaction conditions but with only 40% conversion and 90% selectivity to glycerol carbonate (entry 1, Table 5). In presence of Zn-based catalyst, glycerol conversion enhanced by twofold (87%) with almost complete selectivity towards GC. It was also observed that the parent MCM-41 showed 51% conversion of glycerol, as the OH groups of surface silanol can activate the urea molecule as shown in Scheme 2 (step I) [40]. In step II, Lewis basic sites of ZnO can activate glycerol molecule, which is then prone to the attack on carbonyl carbon of urea with simultaneous release of 1 mol of NH3. In the next step (III) secondary –OH of activated glycerol again attacks over carbonyl carbon of urea to form five membered cyclic glycerol carbonate with the loss of second molecule of NH3. In the last step IV of the reaction pathway, the catalyst surface was regenerated by the release of GC. This suggests that the ZnO and MCM-41 play cooperative role in glycerol carbonylation reaction. This was confirmed by control experiment with only bare ZnO which gave lower glycerol conversion of 68% compared to that of 5% Zn/MCM-41 (78%) with GC selectivity of 98% (entry 3, Table 5).
Conclusion
Among the several acidic supports (MCM-41, SBA-15, SiO2, Al2O3, ZrO2 and s-ZrO2) studied for ZnO catalyst for the glycerol-urea carbonylation, MCM-41 supported Zn catalyst exhibited the highest activity and almost complete selectivity to GC. Increased Zn loading in the rang of 2–10% on MCM-41, resulted in decrease in surface area from 692 to 498 m2 g−1 confirming well dispersed ZnO within the mesoporous structure of silicalite MCM-41which also affect the ordering of the mesoporous structure evident from the XRD analysis. The increased dispersion of Zn with increase in loading has directly reflected in the enhancement of GC yield from 57 to 83%. Interestingly, acidity/basicity ratio was found to be constant (~0.8) for Zn loading in the range of 2–10% while, the glycerol conversion increased substantially with increase in Zn loading irrespective of the acid/base ratio. Glycerol conversion increased linearly from 63 to 82% with increase in active metal loading from 2 to 5% due to higher availability of Lewis acid–base sites generated from ZnO. While, the GC selectivity marginally increased from 92 to almost complete (98%) for >2% Zn loading. Glycerol conversion and GC selectivity were found to be quite sensitive to glycerol to urea mole ratios. For higher mole ratio (2) of glycerol to urea, glycerol conversion dropped down to 44% while, for lower ratio of 0.5, GC selectivity was only 58% with the formation of urethanes as the by-products. In a proposed reaction pathway, OH groups of silanol are shown to activate the urea molecule while Lewis basic sites of ZnO can activate glycerol molecule, which is then prone to attack on carbonyl carbon of urea followed by cyclisation and generating two molecules of NH3.
In a batch operation, glycerol conversion as high as 78% with almost complete selectivity to GC could be achieved at 140 °C; however, a lower glycerol conversion of 55% was achieved even at higher temperature of 200 °C, in a continuous operation over the same catalyst. This was because of ~5 times lower residence time in a continuous operation than that in a batch operation. The activation energy calculated from Arrhenius plot was found to be 39.82 kJ mol−1 K−1. Hence, the batch operation could be better, giving fivefold higher productivity of GC as compared to the continuous for carbonylation of glycerol with urea.
References
BP Statistical Review of World Energy June (2012). http://www.bp.com/statisticalreview
Zhou CH, Beltramini JN, Fan YX, Lu GQ (2008) Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev 37:527–549
Meessen JH, Petersen H (2000) Ammonia and Fertilizers. Wiley-VCH Verlag GmbH & Co. KGaA, Wrocław, Poland
Sakakura T, Choi JC, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107:2365–2387
Du Y, Cai F, Kong DL, He LN (2005) Organic solvent-free process for the synthesis of propylene carbonate from supercritical carbon dioxide and propylene oxide catalyzed by insoluble ion exchange resins. Green Chem 7:518–523
Doro F, Winnertz P, Leitner W, Prokofieva A, Muller TE (2011) Adapting a Wacker-type catalyst system to the palladium-catalyzed oxidative carbonylation of aliphatic polyols. Green Chem 13:292–295
Teles JH, Rieber N, Harder W (1994) Preparation of glyceryl carbonate. US patent 5359094
Li J, Gu Y, Guan Z, Mo W, Ni Y, Li T, Li G (2010) Oxidative carbonylation of glycerol to glycerol carbonate by PdCl2 (Phen)/KI. Appl Catal A 386:188–193
Casiello M, Monopol A, Cotugno P, Milella A, Michela M, Ciminale F, Nacci A (2014) Copper(II) chloride-catalyzed oxidative carbonylation of glycerol to glycerol carbonate. J Mol Catal A Chem 381:99–106
Hu J, Gu Y, Guan Z, Li J, Mo W, Li T, Li G (2011) An efficient palladium catalyst system for the oxidative carbonylation of glycerol to glycerol carbonate. Chemsuschem 4:1767–1772
Appaturi JN, Ng EP, Adam F (2014) Solid imidazolium amide catalysts for the solvent free synthesis of glycerol carbonate. J CO2 Util 6:69–74
Alvarez MG, Pliskova M, Segarra AM, Medina F, Figureas F (2012) Synthesis of glycerol carbonates by transesterification of glycerol in continuous system using supported hydrotalcites as catalyst. Appl Catal B 113–114:212–220
Aresta M, Dibenedetto A, Nocito F, Ferragina C (2009) Valorization of bio-glycerol: New catalytic materials for the synthesis of glycerol carbonate via glycerolysis of urea. J Catal 268:106–114
Vieville C, Yoo JW, Pelet S, Mouloungui Z (1998) Synthesis of glycerol carbonate by direct carbonatation of glycerol in supercritical CO2 in the presence of zeolites and ion exchange resins. Catal Lett 56:245–247
Fukuda Y, Yamamoto Y (2009) Method for producing glycerol derivative. JP Patent 023930
Kondawar SE, Rode CV (2017) Tandem synthesis of glycidol via transesterification of glycerol with DMC over Ba-mixed metal oxide catalysts. ACS Sustain Chem Eng 5:1763–1774
Ochoa-Gomez JR, Gomez-Jimenez-Aberasturi O, Maestro-Madurga B, Pesquera- Rodriguez A, Ramirez-Lopez C, Lorenzo-Ibarreta L, Torrecilla-Soria J, Villaran-Velasco MC (2009) Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: catalyst screening and reaction optimization. Appl Catal A 366:315–324
Mouloungui Z, Yoo JW, Gachen CA, Gaset A, Vermeersch G (1996) Process for the preparation of glycerol carbonat from glycerol and a cyclic organic carbonat, especially ethylene or propylene carbonate. E P Patent 96-390003 739888
Li J, Wang T (2011) On the deactivation of alkali solid catalysts for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. React Kinet Mech Catal 102:113–117
Okutsu M, Kitsuki T (2000) Process for the preparation of glycerol carbonate. WO Patent JP 050415
Kumar P, With P, Srivastava VC, Glaser R, Mishra IM (2015) Glycerol carbonate synthesis by hierarchically structured catalysts: catalytic activity and characterization. Ind Eng Chem Res 54:12543–12552
Sasa T, Okutsu T, Uno M (2008) Method for producing glycerol carbonate. JP Patent 285457
Sasa T, Okutsu T, Uno M (2009) Method for producing glycerol carbonate. JP Patent 067689
Yoo JW, Mouloungui Z (2003) Catalytic carbonylation of glycerin by urea in the presence of zinc mesoporous system for the synthesis of glycerol carbonate. Stud in Surf Sci Catal 146:757–760
Climent MJ, Corma A, De Frutos P, Iborra S, Noy M, Veltyand A, Concepcion P (2010) Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid–base pairs. J Catal 269:140–149
Rubio-Marcos F, Calvino-Casilda V, Banares MA, Fernandez JF (2010) Novel hierarchical Co3O4/ZnO mixtures by dry nanodispersion and their catalytic application in the carbonylation of glycerol. J Catal 275:288–293
Wang L, Ma Y, Wang Y, Liu S, Deng Y (2011) Efficient synthesis of glycerol carbonate from glycerol and urea with lanthanum oxide as a solid base catalyst. Catal Commun 12:1458–1462
Hammond C, Lopez-Sanchez J, Rahim M, Dimitratos N, Jenkins R, Carley A, He Q, Kiely C, Knight D, Hutchings G (2011) Synthesis of glycerol carbonate from glycerol and urea with gold-based catalysts. Dalton Trans 40:3927–3937
Srikanth A, Viswanadham B, Kumar VP, Anipindi NR, Chary VRK (2016) Synthesis and characterization of Cs-exchanged heteropolyacid catalysts functionalized with Sn for carbonolysis of glycerol to glycerol carbonate. Appl Petrochem Res 6:145–153
Kondawar SE, Potdar AS, Rode CV (2015) Solvent-free carbonylation of glycerol with urea using metal loaded MCM-41 catalysts. RSC Adv 5:16452–16460
Zhao D, Feng J, Huo Q, Molish N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Alarcon EA, Villa AL, de Correa CM (2009) Characterization of Sn- and Zn-loaded MCM-41 catalysts for nopol synthesis. Micro Meso Mater 122:208–215
Jiang Q, Wu ZY, Wang YM, Cao Y, Zhou CF, Zhu JH (2006) Fabrication of photoluminescent ZnO/SBA-15 through directly dispersing zinc nitrate into the as-prepared mesoporous silica occluded with template. J Mater Chem 16:1536–1542
Zhong S, Liang L, Liu M, Liu B, Sun J (2015) DMF and mesoporous Zn/SBA-15 as synergistic catalysts for the cycloaddition of CO2 to propylene oxide. J CO2 Util 9:58–65
Sauer J, Marlow F, Schuth F (2001) Simulation of powder diffraction patterns of modified ordered mesoporous materials. Phys Chem Chem Phy 3:5579–5584
Hazrati N, Abdouss M, Vahid A, Miran Beigi AA, Mohammadalizadeh A (2014) Removal of H2S from crude oil via stripping followed by adsorption using ZnO/MCM-41 and optimization of parameters. Int J Environ Sci Technol 11:997–1006
Turco M, Bagnasco G, Cammarano C, Senese P, Costantino U, Sisani M (2007) Cu/ZnO/Al2O3 catalysts for oxidative steam reforming of methanol: the role of Cu and the dispersing oxide matrix. Appl Catal B Envi 77:46–57
Silvestre-Albero J, Sepulveda-Escribano A, Rodrıguez Reinoso F (2008) Preparation and characterization of zinc containing MCM-41 spheres. Micro Meso Mater 113:362–369
Turney TW, Patti A, Gates W, Shaheen U, Kulasegaram S (2013) Formation of glycerol carbonate from glycerol and urea catalysed by metal monoglycerolates. Green Chem 15:1925–1931
Zhang X, Zhao N, Wei W, Sun Y (2006) Chemical fixation of carbon dioxide to propylene carbonate over amine-functionalized silica catalysts. Catal Today 115:102–106
Acknowledgements
One of the authors SEK is thankful to the Council of Scientific and Industrial Research, New Delhi, for its financial support for the award of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kondawar, S.E., Mane, R.B., Vasishta, A. et al. Carbonylation of glycerol with urea to glycerol carbonate over supported Zn catalysts. Appl Petrochem Res 7, 41–53 (2017). https://doi.org/10.1007/s13203-017-0177-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-017-0177-2