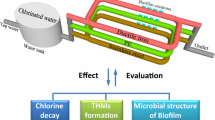
Abstract
In recent years, extensive laboratory-scale research has focused on the biofilm formation, leaching, and migration of organic chemical compounds from plastic pipes into drinking water. This research has been conducted on an existing distribution network in three rural villages near the dead ends to study the small diameter pipes associated with stagnant or low flow conditions. The distribution network was constructed more than ten years ago using unplasticized polyvinyl chloride pipes. Samples of water, soil, and aged pipes have been collected and tested in the laboratory. Results showed a heavy biofilm formed on the inner surfaces of the uPVC pipes. The biofilm has resulted in high concentrations of trihalomethanes, total organic carbon, and PAHs in the drinking water. The predominant PAHs were the acenaphthylene followed by the naphthalene, while no PAHs have detected at the source. The SEM images showed deterioration of pipe walls, swelled parts, and pores. The EDX confirms the migration of some elements including O, Cl, Ca, Ti, Mg, Fe and K due to the biodegradation of the pipe material and the Ca/Zn stabilizers. However, PAHs released from the plastic pipes into the flowing water are caused by metabolic activities. In addition, results showed that the surrounding soil has classified as having low organic content. Hence, uPVC pipes need protection or change in the manufacturing processes to reduce their hazards in distribution networks over time.
Similar content being viewed by others
Introduction
Worldwide, operators face challenges that are acting as obstacles to the sustainability of quality deliverables to consumers. After the construction of water supply systems, continuous research, follow-up and update are the backbone for successful operation. Small water distribution systems may have less monitoring as well as preventive maintenance rather than reactive maintenance because of some kinds of invisible problems occurring through the years in the system without leakage or breaks or direct observations.
The use of un-Plasticized Polyvinyl Chloride (uPVC) pipes has increased due to their lightweight, low cost, ease of installation, besides, having no corrosion problems like their metallic and concrete counterparts (Manuel et al. 2007; Whelton and Nguyen 2012). The pipe material is not pure PVC resin but made of a mixture of PVC resin and several additives such as colorants, stabilizers, mold release agents, lubricants, and antioxidants that may represent up to 40% of the final product (BOHNET 2014).
However, most uPVC manufacturers use the Ca/Zn stabilizers instead of the Pb-based stabilizers to avoid the Pb hazards in drinking water. Stabilizers are essential to prevent chain reaction and increase the PVC resistance, resistance to daylight, weathering, and heat aging. Ca/Zn stabilizers are containing calcium stearate 10–12%, zinc stearate 4–5%, beta-diketone 8–10%, phosphite ester 4–30%, antioxidant 12–15% mainly carboxylic groups, brucite 9–16%, PE wax 13–19%, epoxidized linseed oil 4–10%, auxiliary stabilizer 1–3%. Mostly, the poly aromatic hydrocarbons (PAHs) are mainly involved in the manufacturing process of polymeric or uPVC pipes as antioxidants and lubricants, especially the long-chain carboxylates (C12) such as the acenaphthylene (C12H8) and naphthalene (C10H8) (BOHNET 2014).
The International Agency for Research on Cancer classifies some PAHs as known, possibly, or probably carcinogenic to humans. Among these is benzo[a]pyrene, naphthalene, chrysene, benz[a] anthracene, benzo[k]fluoranthene, and benzo[b] fluoranthene (IARC 2010).
Leaching problems of organic chemicals and hydrocarbons from the plastic pipes into the water have been proven in previous research (Skjevrak et al. 2003, 2005; Mao 2008; Whelton and Nguyen 2012; Shaikh et al. 2019). In addition, the formation of biofilm lining the inner surface of pipes has been studied by several researchers (Skjevrak et al. 2005; Tsvetanova 2006; Manuel et al. 2007; Richardson and Edwards 2009; Rozejet et al. 2014; Biedroń et al. 2017). However, Whelton and Nguyen (2012) concluded that biofilm formation affects contaminant migration, but the scale of this effect is not well documented. On the other hand, the research emphasized that biofilm formation on the surface of uPVC pipes has been found with a higher density compared to the other pipe materials (Cloete et al. 2003; Manuel et al. 2007).
The biofilm formation in the water distribution system consists of several stages, including surface conditioning, irreversible attachment, colonization, and detachment (Manuel et al. 2007; Biedroń et al. 2017). On the other hand, biofilm removal from existing drinking water pipelines is difficult by flushing using chlorinated water (Dueterelo et al. 2016; Van Bel et al. 2019). In addition, recent researchers (Dueterelo et al. 2016; Suprajaa et al. 2017; Calero et al. 2021) reported that the genera found in the biofilm community were Bacillus, Pseudomonas, and Cyanobacteria, in addition to a self-produced matrix of extracellular polymeric substances, including polysaccharides, proteins, and lipids. Moreover, Mixotrophic Cyanobacteria and microalgae can utilize the PAHs including naphthalene, 1-methyl naphthalene, 2-methyl naphthalene, acenaphthene, phenanthrene, fluorene, pyrene, anthracene, fluoranthene, chrysene, benzo(b)fluoranthene, benzo(a)anthracene, benzo(k)fluoranthene, and benzo(a)pyrene (Subashchandrabose et al. 2013).
Meanwhile, the pipe material plays a vital role in biofilm formation due to surface roughness, adhesives, plasticizers, stabilizers, lubricants, and resins involved in the pipe manufacturing process, which can be a source of nutrients for bacteria (Traczewska and Sitarska 2009). This corresponds to (Hua and Wang 2014; Abdel-Shafy and Mansour, 2016), who reported that hydrocarbons are adsorbed on the surface of microbial cells and can be transported across the membrane into the cells mainly through passive transport or active transport.
However, understanding the contaminant migration from these materials affecting drinking water quality is necessary, as well as more work is needed to identify the contaminants, their quantities, occurrence in water distribution networks, and the processes that control their migration from polymer pipes (Whelton and Nguyen 2012). Also, they highlighted that much of the work only represents migration testing results for new polymer pipes at exposure times (1 h. to greater than two years), while drinking water regulations can be based on long-term exposure (70 years).
This research can be considered one of the few field investigations conducted on an existing distribution network in service for more than ten years. The research has included three rural villages at the dead ends of a distribution network fed by one source. The main reasons for conducting the study near the dead ends were to focus on small diameter pipes and stagnant water or low flow condition.
Mainly, the objective of this research was to study the biofilm formation on the inner surface of the aged uPVC pipes as a reason for the leaching and migration of chemical compounds into the water. Accordingly, this research will contribute to developing new solutions, protection methods, or even modifying the manufacturing processes to reduce the hazards of using the uPVC pipes in drinking water distribution networks.
Materials and methods
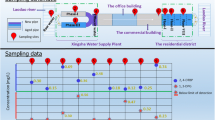
This research has been carried out in the field for a year in three rural villages at different locations, supplied by a conventional water treatment plant, as shown in Fig. 1, to increase the reliability and simulation of environmental conditions. The preferred research technique for the field investigation is the experimental quantitative research approach that implies postulating hypotheses, doing quantitative experiments, and then either sustaining or rejecting the assumptions. Key assumptions of the present research were the investigation of the aged uPVC pipes at the dead ends to ensure the depletion of residual chlorine and ease the detection of low concentrations in stagnant or low flow conditions, and the biofilm formation on the inner surface.
The uPVC pipes specifications
The nominal diameter of the uPVC pipes (outside diameter) is 110 mm (4 inches) at the dead ends with a working pressure of 10 bars and a wall thickness of 5.60 mm. The uPVC pipes in the present study have been manufactured according to the international specifications of ISO 4422/EN1452 uPVC water mains and are equivalent to the American Public Health Association; American Water Works Association; Water Environment Federation (2019) C900, Class 200.
Measurement of pipe wall thickness
The average thickness of the pipe wall was measured using a vernier caliper for the new and aged pipes three times for each sample at different locations along the pipe perimeter. The accuracy of the used vernier caliper was up to 0.10 mm.
Sampling of water
Samples were collected monthly from raw water intake before the treatment plant, treated water from the rising main at the high lift station before distribution, and from the dead ends. More than (24) samples of raw and treated water were collected routinely by the plant operators for inspection, in addition to about (36) samples collected from the dead ends for analysis in the central laboratory. The reasons for collecting samples from the distribution system at the dead ends were to ensure the long hydraulic retention time and ease the detection of low concentrations of the PAHs due to the accumulation of migrant chemical elements from the uPVC pipes into the water.
The PAHs have been measured using gas chromatography (GC) followed by mass spectrometry (MS) according to the EPA 550/1990 method, including naphthalene, acenaphthene, and phenanthrene, anthracene, fluoranthene, acenaphthylene, and other aromatic compounds at the source and at the dead ends. The preservation technique for the collected samples has been followed, and water quality parameters have been tested according to the APHA (23rd edition) as listed in Table 1.
Microbiological and biofilm analysis
Three rings were cut from the existing pipelines as samples at the dead ends three times throughout the study. After that, three pieces of 1 cm2 have cut from each for the biofilm analyses. The biofilm was scraped from the surface of each piece and investigated by counting the viable cells. The microbiological examinations were according to the APHA (23rd edition). However, Cyanobacteria was determined as the main biofilm constituents using traditional PCR methods for obtaining accurate results. Meanwhile, two methods were applied to determine the heterotrophic plate count (HPC), including the nutrient and R2A agar media. However, the R2A agar media is recommended over the nutrient agar media to determine the heterotrophic plate count (HPC) in environmental samples such as drinking water or groundwater (Stomp 2008). Table 2 illustrates the measured biological characteristics and the followed methods.
The characteristics of the raw and treated water
The average values of the measured parameters for the raw and treated water are listed in Table 3. However, the effluent from the treatment plant complies with regulatory standards.
SEM and EDX
The scanning electron microscopy (SEM) and the energy-dispersive X-ray (EDX) have been applied to (9) samples of the aged uPVC pipes three times throughout the study. On the other hand, a new uPVC pipe from the same manufacturer has been tested in the same manner for comparison as a control blank. Three workpieces have been cut to 1 cm2 from each sample and prepared for electron microscopy, according to standard methods, with gold sputter coating. SEM analyses were applied to investigate the biofilm, the swelled parts of the uPVC pipes, the surface roughness, and the sizes of pores.
Soil sampling
Samples were collected from the soil surrounding the aged uPVC pipes to determine the organic contaminants. Samples were taken three times through the study period, each weighing one kilogram. Samples were collected and preserved according to standards of the ISO 10694–1/1995 ES 5391–2014. Concentrations of organic contaminants, including carbon (C), nitrogen (N), and hydrogen (H), were measured by the (NC) Soil Analyzer model Thermo scientific flash 2000. SN. 2015F0028.
Results and discussion
The results showed high correspondence to the previous research regarding pipe aging, biofilm formation, leaching, and migration of chemical compounds into drinking water. The following are the critical results summarized from the field study.
The aging of uPVC pipes
According to the results obtained from the SEM and EDX, the inner surface of the aged uPVC pipe has deteriorated in terms of increased surface roughness, degradation, and pores of the inner surface compared to a smoother surface of the new pipe sample, as shown in Fig. 2a and b.
The energy-dispersive X-ray (EDX) results
The EDX analysis showed high Cl, Ca, and K peaks linked to the pipe material in Fig. 3a in the case of the new pipe. While the other low values of K, Al, Fe, Mg, and Na are due to the degradation of the antioxidants, deposition of inorganics, and bacterial secretions in correspondence with (Abrusci et al. 2011) who found that efficient development of biodegradation processes for polyethylenes with calcium-stearate doped polyethylenes. However, the presence of O, Cl, Ca, Ti, and K in high peaks in the case of the aged pipes (Fig. 3b) confirms the migration of these elements due to the degradation of the pipe material since the stabilizers are mainly based on Ca/Zn alkyl carboxylates. These results demonstrate the degradation of resins and stabilizers in the pipe material. However, the higher oxygen value (O) in Fig. 3b is probably due to the degradation and hydrolyzation of the carboxylic groups involved in the Ca/Zn stabilizer. The main reason for this is the presence of calcium stearate as the main component of the Ca/Zn stabilizer is biodegradable by most bacteria to water and calcium oxides or carbonate.
Biofilm formation on the internal surface
According to the SEM images, thick biofilm has been formed on the internal surface of the aged uPVC pipes due to the increased surface roughness and the existence of large pores up to 15 μm as shown in Fig. 4a and b. These results are consistent with the primary factors reported by Biedroń et al. (2017) for biofilm formation and increasing the adhesion between the microorganisms and the pipe surface.
The biofilm constituents were Cyanobacteria, Bacillus, Micrococcus, and Pseudomonas matching the results of previous research (Dueterelo et al. 2016; Suprajaa et al. 2017; Calero et al. 2021). However, Cyanobacteria is the base of the formation of the mucilaginous layer and was too numeric to count, which correlated with Dueterelo et al. (2016). Moreover, the algal counts in the water samples at the dead ends have reached up to 840 counts/ml exceeding the regulatory standards of 1 count/ml, due to the detachment of the blue-green algae (Cyanobacteria) from the pipe wall into the flowing water. Yet, most of these gram-positive bacteria are rapidly degrading the low molecular weight PAHs (Lu et al. 2019). The bacterial genera found in the formed biofilm correspond to the reported genera by several researchers (Dueterelo et al. 2016; Suprajaa et al. 2017; Calero et al. 2021). Noteworthy, Cyanobacteria and microalgae are highly adaptive and can grow autotrophically, heterotrophically, or mixotrophically (Subashchandrabose et al. 2013), which explains the discussion by Dueterelo et al. (2016) on why these photosynthetic bacteria are commonly present in biofilms in drinking water distribution systems, while it has suggested that they might have an alternative non-phototrophic lifestyle.
The bacterial genera found in the scraped biofilm are involved in PAH degradation, as noted by Lu et al. (2019), which explains the release of the PAHs from the pipe surface into the water. In addition, the release of naphthalene in the metabolic byproduct due to the degradation of the organic pollutants of industrial origin by Cyanobacteria (Subashchandrabose et al. 2013). Figure 5 illustrates the biofilm role in releasing the PAHs from the uPVC pipes into the flowing water.
Deterioration of water characteristics
The total organic carbon (TOC) and trihalomethanes (THMs) have been measured in the raw water, treated water, and dead ends. Results showed that the TOC ranged from less than 3.20 mg/l at the high lift station to 4.38 mg/l at the dead ends, while the average TOC in the raw water was 4.60 mg/l, as illustrated in Fig. 6. Consequently, the THMs increased from (40–60) ppb at the high lift station to (120–180) ppb at the dead ends, exceeding the standard limits of 80 ppb, as shown in Fig. 7, while there were no THMs have been detected in the raw water.
Mainly, the high formation rates of both TOC and THMs are pertinent to the biofilm formation on the internal surfaces along with the distribution network. Thus, corresponding to Abokifa et al. (2016), who reported that biofilms are ubiquitous in the drinking water pipes, the chlorination of the microbial carbon associated with the biofilm contributes to the total disinfection by-products (DBPs) formation with distinct mechanisms from those formed from precursors derived from natural organic matter (NOM).
Leaching and migration of aromatic hydrocarbons from pipes
Table 4 presents the maximum, minimum, and average values of the measured PAHs at each dead-end. However, the predominant PAH is acenaphthylene, followed by naphthalene. Otherwise, the remaining PAHs have minor concentrations below the detection level. In Fig. 8, the average values of the total PAH concentration were 494.77, 134.00, and 395.33 ng/l at dead-end 1, 2, and 3, respectively. The average acenaphthylene ranged from 86 to 477 ng/l, and the average naphthalene ranged from 46 to 98 ng/l at the dead ends. Meanwhile, no PAHs have been detected at the source or in raw water. However, both PAHs affect human health at low concentrations or consumption (IARC 2010; Karyab 2013; Abdel-Shafy and Mansour, 2016).
Degradation of pipes material and thickness reduction
According to the measured pipe wall thickness for all pipe samples, the average thickness has decreased from 5.60 to 4.80 mm, due to the biodegradation of the antioxidants as well as stabilizers in the surface layer, complying with Colin et al. (2009), who reported the migration of antioxidants from the inner surface layer into the water. Also, Colin et al. (2009) have reported failures with brittle cracking were observed in natural aging after exposure times of 5 to 15 years, confirming the results of this research and emphasizing the failure of the aged pipes far before the expected lifetime.
The difference between the new and aged pipe surfaces is clear, as shown in Fig. 9. The new pipe surface in Fig. 9a shows a smoother surface. However, in Fig. 9b, the protruded resins due to the migration of the superficial layer increased the surface roughness and resulted in the wall thickness reduction, thus, demonstrating the migration and leaching of organic compounds from the pipe into the drinking water.
Deterioration of the external surface
The analyses of the surrounding soil showed that the average carbon content was 10.6 gm/kg, the average nitrogen concentration was 1.33 gm/kg, and hydrogen was 13.58 gm/kg. According to these results, the surrounding soil has classified as having low organic content. However, samples taken to scan the external surface by the SEM and EDX showed that the situation of the outer surface was not better than the inner surface in terms of swelled parts, surface roughness, large pores, and formation of biofilm, as shown in Fig. 10.
Noteworthy, during the maintenance and replacement of a broken uPVC pipe, it has been observed that it has dark random streams on its outer surface, as shown in Fig. 11. Most logically, the dark streams, the swelled parts, and the changing of the pipe material characteristics are due to microbial activities, besides the contact between the organic contaminants in soil and the outer pipe surface.
Conclusions
The biofilm formation in distribution networks has been investigated by many researchers (Stomp 2008; Revetta et al. 2010; Dueterelo et al. 2016; Suprajaa et al. 2017; Calero et al. 2021), especially the uPVC pipes. Most researchers have focused on biofilm formation, identification of bacterial genera, deterioration of water quality due to biofilm detachment, the impact of limiting nutrients at the water source, and cleaning by flushing chlorinated water. However, no researchers have realized that the pipe material contains the required nutrients for the bacteria, which hydrolyses the pipe material and releases chemical compounds into the drinking water, especially the PAHs. Therefore, controlling the biofilm formation by limiting the nutrients at the water source is difficult, considering the low effectiveness of chlorine in removing biofilm or preventing biofilm regrowth.
The results obtained from the present research emphasize the failure of the aged uPVC pipes after (10) years in service, far before the expected lifetime of more than (40) years. The biofilm formation, leaching, and migration of chemical compounds, including the polycyclic aromatic hydrocarbons from the uPVC pipe, and high formation rates of both TOC and THMs have demonstrated to constitute highly potential risks over time, after installation and mainly by the microbial metabolic activities.
In general, the regulatory standards must have maximum allowable limits for each separate PAH and the organic industrial pollutants such as naphthalene and acenaphthylene, which are not regulated, to help the operators of distribution systems to recognize the migration and leaching problems from plastic pipes in the small systems and save public health. Moreover, standard microbial tests should be applied to the new pipes to determine the microbial effect on the pipe material in the long term.
Availability of data and materials
All presented data in this manuscript are available and will be sent upon request.
References
Abdel-Shafy H, Mansour M (2016) A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt J Petrol 25(1):107–123. https://doi.org/10.1016/j.ejpe.2015.03.011
Abokifa AA, Yang YJ, Lo CS, Biswas P (2016) Investigating the role of biofilms in trihalomethane formation in water distribution systems with a multicomponent model. Water Res. https://doi.org/10.1016/j.watres.2016.08.006
Abrusci C, Pablos JL, Corrales T, López-Marin J, Marín I, Catalina F (2011) Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int Biodeterior Biodegradation 65:451–459. https://doi.org/10.1016/j.ibiod.2010.10.012
American Public Health Association; American Water Works Association; Water Environment Federation (2017) Standard Methods for the Examination of Water and Wastewater, 23rd edn. American Public Health Association, Washington, D.C
Biedroń I, Traczewska T, Konieczny T, Płaza G (2017) Characterization of biofilms from selected synthetic materials used in water distribution system. J Ecol Eng 18:284–293. https://doi.org/10.12911/22998993/67850
BOHNET M (2014). Ullmann's encyclopedia of industrial chemistry. http://onlinelibrary.wiley.com/book/https://doi.org/10.1002/14356007
Calero PC, Husband S, Boxall J, Del Olmo G, Soria-Carrasco V, Maeng SK, Douterelo I (2021) Intermittent water supply impacts on distribution system biofilms and water quality. Water Res 1(201):117372. https://doi.org/10.1016/j.watres.2021.117372 (Epub 2021 Jun 17 PMID: 34198200)
Cloete T, Westaard D, van Vuuren S (2003) Dynamic response of biofilm to pipe surface and fluid velocity. Water Sci Technol 47:57–59. https://doi.org/10.2166/wst.2003.0280
Colin X, Audouin L, Verdu J, Rozental-Evesque M, Rabaud B, Martin F, Bourgine F (2009) Aging of polyethylene pipes transporting drinking water disinfected by chlorine dioxide Part II—Lifetime prediction. Polym Eng Sci 49(7):1429–1437
de Nikki Bel, Hornstra LM, Anita Veen, Gertjan Medema (2019) Efficacy of flushing and chlorination in removing microorganisms from a pilot drinking water distribution system. Water 11:903. https://doi.org/10.3390/w11050903
Douterelo I, Jackson M, Solomon C, Boxall J (2016) Microbial analysis of in situ biofilm formation in drinking water distribution systems: implications for monitoring and control of drinking water quality. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-015-7155-3
Hua F, Wang H (2014) Uptake and trans-membrane transport of petroleum hydrocarbons by microorganisms. Biotechnol Biotechnol Equip 28:165–175. https://doi.org/10.1080/13102818.2014.906136
IARC (2010) International Agency for Research on Cancer, Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures, Monogr Eval Carcinog Risks Hum 92:765–771
Karyab H, Yunesian M, Nasseri S, Mahvi A, Ahmadkhaniha R, Rastkari N, Nabizadeh R (2013) Polycyclic aromatic hydrocarbons in drinking water of Tehran Iran. J Environ Health Sci Eng 11:25. https://doi.org/10.1186/2052-336X-11-25
Lu C, Hong Y, Liu J, Gao Y, Ma Z, Yang B, Ling W, Waigi MG (2019) A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.05.044
Manuel C, Nunes OC, Melo L (2007) Dynamics of drinking water biofilm in flow/non-flow conditions. Water Res 41:551–562. https://doi.org/10.1016/j.watres.2006.11.007
Mao, F (2008) "Permeation of hydrocarbons through polyvinyl chloride (PVC) and polyethylene (PE) pipes and pipe gaskets". Retrospective Theses and Dissertations. 15797. https://lib.dr.iastate.edu/rtd/15797
Revetta R, Pemberton A, Lamendella R, Iker B, Domingo J (2010) Identification of bacterial populations in drinking water using 16S rRNA-based sequence analyses. Water Res 44:1353–1360. https://doi.org/10.1016/j.watres.2009.11.008
Richardson, R., and Edwards, M. A., 2009. Vinyl chloride and organotin stabilizers in water contacting new and aged PVC pipe, Final report #2991. Denver, CO: Water Research Foundation
Rozejet A, Cydzik KA, Kowalska B, Kowalski D (2014) Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-014-1761-6
Shaikh MM, Mohd Hanafiah MO, Basheer A (2019) Leaching of organic toxic compounds from PVC water pipes in Medina Al-Munawarah Kingdom of Saudi Arabia. Processes 7(10):641
Skjevrak I, Due A, Gjerstad K, Herikstad H (2003) Volatile organic components migrating from plastic pipes, (HDPE, PEX and PVC) into drinking water. Water Res 37:1912–1920. https://doi.org/10.1016/S0043-1354(02)00576-6
Skjevrak I, Lund V, Ormerod K, Herikstad H (2005) Volatile organic compounds in natural biofilm in polyethylene pipes supplied with lake water and treated water from the distribution network. Water Res 39:4133–4141. https://doi.org/10.1016/j.watres.2005.07.033
Stomp Maayke (2008) Colourful Coexistence: A New Solution to the Plankton Paradox. Universiteit van Amsterdam, Amsterdam
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2013) Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ Int 51:59–72. https://doi.org/10.1016/j.envint.2012.10.007
Suprajaa N, Prasad T, Isolation Davida (2017) Characterization and sequencing of biofilm bacterial consortia from drinking water PVC pipelines and their influence on corrosion of mild Steel. COJ Nurse Healthcare. https://doi.org/10.31031/COJNH.2017.01.000512
Traczewska TM, Sitarska M (2009) Development of biofilm on synthetic polymers used in water distribution. Environ Prot Eng 35:151–160
Tsvetanova Z (2006) Study of biofilm formation on different pipe materials in a model of drinking water distribution system and its impact on microbiological water quality. https://doi.org/10.1007/978-1-4020-5098-5_46
Whelton A, Nguyen T (2012) Contaminant migration from polymeric pipes used in buried potable water distribution systems: a review. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2011.627005
Funding
The author received no funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was not published before or submitted to another journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fadel, H.A. Contamination from plastic pipes in small systems: migration and leaching. Appl Water Sci 12, 231 (2022). https://doi.org/10.1007/s13201-022-01751-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01751-y