Abstract
Uranium concentration in the ground water samples from the district of Tiruvannamalai, Tamil Nadu, was measured using an LED fluorimeter. All the samples were qualified as potable water from the radiological perspective. Though some samples showed mild chemical toxicity, they are still safe for ingestion. Different risk coefficients were calculated, and they were compared with recommended safety limits specified by various agencies. Software tools such as QGIS 15, GraphPad Prism 8 and Surfer 15 were employed for developing maps and plots.
Similar content being viewed by others
Introduction
Water is an essential resource and a boon to the mankind. Due to rapid industrialisation and urbanisation, the quality of water is constantly deteriorating. The environmental changes have also made the availability of ground water very scarce in arid and semi-arid regions. Tiruvannamalai, a district in the north eastern part of Tamil Nadu, India, is relatively a dry region due to lack of surface water reservoirs and flowing rivers. Hence, the local population is largely dependent on ground water for cooking, drinking and agriculture. Due to a considerable drop in the ground water levels in the recent years, water obtained from ground water sources such as wells and borewells pose a higher risk of contamination due to rock water interaction. Contamination to some degree can also be attributed to extensive use of fertilizers by the peasant farmers in this district.
Materials and methods
Study area: location, climate and geology
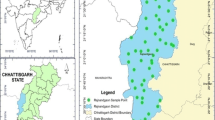
The study area for this research is the district of Tiruvannamalai spanning over an area of 6188 km2 located at 11.55° and 13.15° north latitude and 78.20° and 79.50° east longitude (Fig. 1). The average population is 2,464,875 according to census 2011, with over 63% of the working population engaged in agriculture (DSH 2018). The average annual rainfall is reported to be only 813.1 mm (Report 2014) and is regularly prone to drought during summer. To make matters worse, there are no perennial rivers or water reservoirs available in the district forcing the public and farmers to rely on ground water either from bore wells or open wells.
The digital geospatial layers for the preparation of lithological map were obtained from the Geological Survey of India (GSI). Figure 2 shows the land cover of the district divided into square grids (10.85 km × 10.85 km) using QGIS—open-source mapping software. A location was identified in each grid depending on the availability and approachability of the sampling site. The geological study of this region indicates the presence of igneous and metamorphic rocks in general. The lithological map of the study area in Fig. 3 shows the presence of granites, charnockites, migmatite–Gneiss, Peninsular Gneiss, Sukinda ultramafics and alkaline complexes. Granites and charnockites are well known for the presence of uranium in them, and this provides an ample reason to choose Tiruvannamalai District for this study. The water samples were carefully collected from each site using standard procedures prescribed by ICRP, and after completing the in situ measurements, they were transported to the laboratory for further analysis.
Sample collection
Sample collection containers were polyethylene bottles, each of 1 l capacity. The containers and their lids were washed using a dilute detergent solution and were rinsed thoroughly using 10% nitric acid solution (Virk 2016). The containers were then soaked overnight in a 1:1 diluted nitric acid solution and were further thoroughly dried before taking to them to the sample collection sites (Sar et al. 2018). In the case of a bore well, water was purged out from it for 5 minutes in order to obtain a fresh water sample. Each sample container was rinsed with the same water before it was filled with it. The temperature and GPS location of each sample were noted down in situ, and the container was then labelled neatly before it was transported to the laboratory.
Measurement of physico-chemical parameters
Using a multi-parameter tester designed by Hanna Instruments (HI98194), the TDS, pH and EC of the water sample were determined.
Measurement of uranium concentration
The samples were carefully filtered using a 0.45-µm filter paper and were subject to uranium analysis using an LED fluorimeter (Quantalase Uranium Analyser—QL/LEDF/01) developed by Laser Applications and Electronics Division at the Centre for Advanced Technology, Department of Atomic Energy, Indore, India (Bhangare et al 2013).
The LED fluorimeter causes fluorescence in a sample when it is set to match its excitation condition. A photomultiplier tube is used to detect fluorescence. An ADC converts the signal from the photomultiplier tube into digital pulses. The digital pulses are fed into a microcontroller and are later processed by the built-in computer software.
Each water sample is mixed with 10% of buffer solution (sodium pyrophosphate and orthophosphoric acid) to ensure a uniform yield for all complexes of uranium. 6 ml of the mixture is then poured carefully into quartz cuvette and placed in the LED fluorimeter. A schematic diagram in Fig. 4 shows the analytical procedure for uranium quantification using an LED fluorimeter.
Dose assessment
Chemical toxicity assessment
Lifetime average daily dose
The lifetime average daily dose (WHO 2011) refers to quantity of a substance that is ingested by a person per day per kg of his body weight.
where C—concentration of uranium (µg l−1), DWI—daily water intake (3.7 l for an adult male and 2.7 l for an adult female) (USNAS 2004), EF—exposure frequency (365.25 days) (USEPA 2011), LE—life expectancy (70 years) (USEPA 2011), AT—average exposure time (365.25 × 70 = 25567.5 days) and BW—average body weight (70 kg) (USEPA 1991).
Hazard quotient
The extent to which ingestion of uranium from drinking water can be hazardous to an individual is determined by the hazard quotient (WHO 2011).
where LADD—lifetime average daily dose and RfD—reference dose (0.6 µg/kg and 1.2 µg/kg are the reference doses prescribed by WHO (2011) and AERB 2004, respectively).
Radiological risk analysis
Whole body radiation dose
Annual whole body radiation dose (WHO 2011) is given by Equation 3
where Ac—activity concentration of uranium (Bq/l), DWI—daily water intake (L), EF—exposure frequency (365.25 days) (USEPA 2011) and DCF—dose conversion factor (Sv/Bq). Table 1 shows the age-dependent conversion factors prescribed by ICRP (1995) (ref).
Cumulative whole body dose
Cumulative whole body radiation dose (WHO 2011) is the whole body radiation dose over the life span of an individual.
where LE is the average life expectancy (70 years) (USEPA 2011).
Lifetime cancer risk
Lifetime cancer risk due to the ingestion of uranium contaminated water is given by Equation 5 (USEPA 2011)
where Ac—activity concentration of uranium (Bq/l) and R—risk factor.
Risk factor R is calculated using Equation 6
where RC—risk coefficient (mortality and morbidity) (USEPA 2011) and DWI—daily water intake.
Results and discussion
A total of 63 ground water samples were collected from the district of Tiruvannamalai, Tamil Nadu. Basic physico-chemical parameters such as pH, EC and TDS for all the samples were determined using a multi-parameter tester. Table 2 shows all the 63 samples with their corresponding parameters and their GPS coordinates.
pH
pH is the measure of the concentration of hydrogen ions in a given sample. It measures the relative presence of hydrogen and hydroxyl ions on a 14-point scale ranging from 0 to 14. The pH value is represented in logarithmic units, i.e., an increase or decrease in pH by 1 unit would mean a tenfold increase or decrease in the sample’s acidity. The pH of the samples ranged from 6.75 to 8.67 with a mean value of 8.1. Only samples from locations CHN-W8 (Neepathurai) (8.67) and VEM-W58 (Abdullapuram) (8.53) were found to marginally fall out of the safety range specified in both the WHO (2011) and BIS (2012) guidelines indicating slight alkalinity. Table 3 shows the number of samples qualifying the safety limits of BIS and WHO. Figure 5 shows the distribution of pH in the samples, and Figure 6 shows the spatial distribution in the study area.
EC
Electrical conductivity known as specific conductance is the ability of the sample to allow the passage of electric current through it. Ground water shows higher electrical conductivity due to the presence of dissolved salts due to rock water interaction. Hence, electrical conductivity of ground water in a region will depend largely on its geology with which it interacts. EC of the ground water samples ranged between 1.275 and 96.4 mS cm−1 with the mean value of 29.67 mS cm−1. All the water samples fall within the acceptable BIS limits (BIS 2012). Distribution of EC in the samples and the spatial variation of EC in the study area are shown in Figs. 7 and 8, respectively.
TDS
Water being a good solvent allows the easy dissolution of many inorganic salts and also some organic substances. The TDS measurements of the samples ranged from 233 to 2488 mg/l with a mean value of 790 mg/l. On comparison with the WHO (2011) set water safety guidelines 26 samples were found to be within the acceptable limit of 600 mg/l and 26 samples exceeded it, whereas 11 samples were unpalatable as they exceeded the maximum permissible limit of 1000 mg/l. Considering the BIS (BIS 2012) guidelines 17 samples fell within the acceptable limit (<500 mg/l) and 42 samples exceeded it, whereas 4 samples from Pulivanandal (CHT-W12), Edaipirai (POL-W37), Sodiambakkam (VEM—W60) and Vembakkam (VEM W-61) exceeded the maximum permissible limit of 2000 mg/l. Distribution of TDS in the samples and the spatial variation of TDS in the study area are shown in Figs. 9 and 10, respectively. Table 4 shows the number of samples within and out of the range specified by BIS and WHO.
Concentration of uranium
The concentration of uranium in the samples was determined using an LED fluorimeter. The minimum concentration was recorded to be 0.17 µg/l and the maximum as 43.26 µg/l. The mean concentration was found to be 3.22 µg/l. The permissible limits of uranium concentration in water have been recommended by various agencies such as the WHO (2011), USEPA (2011), ICRP (1995), UNSCEAR (1982) and AERB (2004). In comparison with the WHO set permissible limit of 15 µg l−1, it was observed that 3 samples from Pulivanandal (CHT-W12), Edaipirai (POL-W37) and Sodiambakam (VEM-W60) exceeded the limit. UNSCEAR (UNSCEAR 1982) has set a limit of 9 µg/l which has been exceeded by 5 samples from Pulivanandal (CHT—W12), Veerapanur (JHL—W22), Edaipirai (POL-W37), Sodiambakkam (VEM V60) and Vembakkam (VEM-W61). 30 µg/l is the permissible limit stipulated by USEPA (1982), and only sample from Pulivanandal (CHT-W60) is found to exceed this limit. AERB (AERB 2004) being the only agency with the highest permissible limit of 60 µg/l all the samples easily qualified it. The lowest permissible limit, i.e., 1.9 µg/l, has been recommended by ICRP (1995), only 38 samples fell within the aforesaid limit, and the rest 25 samples exceeded it.
The number of samples that qualified and disqualified the different permissible limits specified by various agencies is shown in Table 5. Figure 11 shows a plot of the frequency distribution of uranium concentration and Figure 12 shows the spatial distribution of uranium in the study area.
The central region on the spatial map showing denser contours indicates higher concentration of uranium. It is observed that the rocks in this region are mainly charnockites which could considerably contribute to elevated levels of uranium concentration. However, other regions of the same rock type show relatively lower concentration. As the region is not an industrial hub, the only possible reason could be the usage of fertilizers in vast agricultural lands
Lifetime average daily dose
The chemical toxicity risk due to the ingestion of uranium through drinking water is assessed by calculating the life time average daily dose. LADD for this study has been calculated (Table 2) separately for adult male and female using the WIR of 3.7 l day−1 and 2.7 l day−1, respectively (USNAS 2004). LADD for adult males varied from a minimum of 0.01 µg kg−1 day−1 to a maximum of 2.29 µg kg−1 day−1 with an average of 0.17 µg kg−1 day−1. On the other hand, LADD in adult females varied from a minimum of 0.01 µg kg−1 day−1 to a maximum of 1.67 µg kg−1 day−1 with an average of 0.12 µg kg−1 day−1. The LADD in adult male and female is plotted in Figs. 13 and 14, respectively. WHO (2011) has specified a threshold limit of 1 µg kg−1 day−1 which is seen to be exceeded by the sample from Pulivanandal (CHT –W12). Spatial distribution of LADD in the study area for adult male and female is also shown in Figs. 15 and 16. The rock formations at Pulivanandal are mainly charnockites and may contribute to elevated levels of LADD. Perhaps the usage of fertilizers is also causing a higher uranium concentration in ground water as they tend to easily seep into the ground water table.
Hazard quotient
The extent to which the ingestion of uranium from ground water can be dangerous is determined by calculating (Table 2) the hazard quotient. The hazard quotient for the samples has been calculated separately for adult males and adult females using two different reference doses (RfD) 0.6 µg kg−1 day−1 and 1.2 µg kg−1 day−1 as recommended by WHO (2011) and AERB (2004), respectively. As per WHO guidelines HQ > 1 is considered chemically dangerous (WHO 2011). It is observed that the HQ of the sample from Pulivanandal (CHT-W12) alone exceeds the stipulated safety limit. The distribution of hazard quotient using WHO and AERB RfDs is presented in Figs. 17 and 18, respectively. Spatial contour maps using both AERB and WHO RfDs are shown in Fig. 19. It is noted the region around Pulivanandal (CHT-W12) shows denser contour lines.
Whole body radiation dose
The absorption dose rate is known to vary for each organ in the human body. The whole body dose rate is the total absorption dose rate of the entire body taking into account the individual absorption rate of each organ over a period of one year. Table 7 shows the variation whole body dose rate for different samples. Statistical parameters along with their corresponding conversion factors pertaining to the whole body dose rate for various age groups are shown in Table 6. Fortunately, all the samples are safe with respect to the WHO’s stipulated threshold limit of 100 µSv/Year (WHO 2011). The violin plot in Fig. 20 shows the distribution in the study area across different age groups. Contour maps in Figs. 21 and 22 show the spatial variation of annual whole body dose in the study area.
Cumulative whole body dose
The total whole body dose received by an individual during his life time is known as cumulative whole body dose. Considering the average span of a typical adult to be 70 years, the cumulative dose rate has been calculated for both adult male and female (Table 7). For adult males, it is found to range from 18.08 µSv to 4600.34 µSv with a mean of 342.54 µSv. On the other hand, for the adult females, it is found to range from 13.19 µSv to 3357.00 µSv with a mean of 249.96 µSv. Frequency distributions plot of cumulative whole body dose for both male and female is shown in the plot (Fig. 23). Spatial variation of cumulative whole body dose is shown in Figs. 24 and 25 for male and female, respectively.
Lifetime cancer risk
The life time cancer risks for mortality and morbidity were calculated for all the samples (Table 7) using the risk coefficients prescribed by USEPA (2011). For the commonly occurring isotopes (omitting the traces), U238, U235 and U234 the risk coefficients of mortality are 1.13 × 10−9, 1.21 × 10−9 and 1.24 × 10−9, respectively. The risk coefficients of morbidity for U238, U235 and U234 are 1.73 × 10−9, 1.88 × 10−9, and 1.91 × 10−9, respectively. The statistical parameters of life time cancer risk (mortality and morbidity) for adult male and female are shown in Tables 8 and 9, respectively. The limit of safety as specified by AERB (AERB 2004) is 1.67 × 10−4, and all the 63 samples qualify to be safe as they all fall within this limit. The USEPA (USEPA 2011) limit is much higher than that of AERB; hence, all the samples easily qualify to be very safe. Violin plots of life time cancer risk (mortality and morbidity) for male and female are shown in Figs. 26 and 27, respectively. The spatial variation of life time cancer risk for U238 (mortality and morbidity) is shown in Fig. 28.
Summary
The ground water samples from 63 locations in the district of Tiruvannamalai have been analysed for palatable and potable. The study area was divided into grids using QGIS, and samples were collected from each grid covering the entire region. A lithological map was obtained from Geological Survey of India’s website (Bhuvan), and the sampling sites were overlaid on the map to determine the type of rock formation at the sampling site. TDS, pH and EC for the samples were determined using a multi-parameter water tester, and the concentration of uranium was found using an LED fluorimeter. Lifetime average daily dose and hazard quotient were calculated to assess the chemical toxicity, whereas whole body radiation dose, cumulative whole body dose and excess cancer risk were calculated to assess the radioactive risk. To study the distribution of parameters in the samples, Violin, Whisker and Scatter plots were drawn. Using Surfer spatial contour maps for each parameter were plotted. Each parameter was compared with the limits of safety specified by different agencies.
Based on AERB guidelines of safety, all the samples showed the concentration of uranium to be below than 60 µg/l. However, some samples exceeded USEPA (2011), WHO (2011), UNSCEAR (1982) and ICRP (1995) limits. Sample from Pulivanandal (CHT-W12) was found to exceed the WHO limit for both LADD and HQ. Carcinogenic risk assessment done using whole body radiation dose, cumulative whole body dose and life time cancer risk revealed that the water samples are potable.
As there are no industries, tanneries or mills in the region around Pulivanandal (CHT-W12), the possible reason for higher concentration of uranium could be attributed to excessive use of fertilizers. Agriculture being the main occupation of the local population, farmers grow short-term crops and apply fertilizers to get better yields. More information could be obtained on conducting a detailed study of the rock formation, soil types and anthropogenic activities in this region.
References
AERB (Atomic Energy Regulatory Board) (2004) Department of Atomic Energy, Drinking Water Specification in India; Government of India
Bhangare RC, Tiwari M, Ajmal PY, Sahu SK, Pandit GG (2013) Laser flourimetric analysis of uranium in water from Vishakapatnam and estimation of health risk. Radiat Protect Environ 36(3):128–132
BIS (Bureau of Indian Standards) (2012) Indian standard drinking water—specification (second revision) IS10500:2012
DSH (District Statistical Handbook) (2018–2019) Department of Economics and Statistics, Tiruvannamalai, Government of Tamil Nadu; released by the District Collectorate, Tiruvannamalai District, Tamil Nadu. Geological Survey of India’s Website, Government of India. http://bukosh.gsi.gov.in/Bhukosh
ICRP (1995) Age-dependent doses to the members of the public from intake of radionuclides—part 5 compilation of ingestion and inhalation coefficients. ICRP Publication 72. Ann. ICRP 26(1)
Report no. ESSO/IMD/HS/R.F. REPORT/02 (2014)/18, Rainfall statistics of India 2013, IndiaMeteorological Department (Ministry of Earth Sciences) by Dr. (Mrs.) Surinder Kaur and M.K. Purohit
Sar SK, Diwan V, Biswas S, Singh S, Sahu M, Jindal MK, Arora A (2018) Study of Uranium levels in underground water of Balod district of Chattisgarh State, India and Assessment of Health Risk. J Hum Ecol Risk Assess 24(3):691–698
UNSCEAR (United Nations Scientific committee on the Effects of Atomic Radiation) (1982) Ionizing radiation: sources and biological effects. New York, United Nations
USEPA (United States Environmental Protection Agency) (1991) “Review of RSC analysis”, Report prepared by Wade Miller Associates, Inc. for the United States Environmental Protection Agency, Washington, DC
USEPA (United States Environmental Protection Agency) (2011) Edition of the drinking water standards and health advisories. EPA 820-R-11-002, Office of water, USEPA, Washington, DC
USNAS (United States National Academy of Science) (2004) Institute of medicine, food and nutrition board dietary reference intakes for water, potassium, sodium, chloride and sulphate. The National Academics Press, Washington, DC
Virk HS (2016) Measurement of concentration of natural uranium in ground waters of Bathinda District (S. Punjab) for the assessment of Annual Effective Dose. Glob J Hum Soc Sci 16:1
WHO (World Health Organisation) (2011) Guidelines for drinking—water quality, 4th edn. World Health Organisation, Geneva
Acknowledgements
The authors are extremely grateful to the Dr. B. Venkataraman, Director IGCAR Kalpakkam, for permitting them to utilize the laboratory for this study.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This paper work does not have any conflict of interest and ethical statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganesh, D., Senthilkumar, G., Eswaran, P. et al. A peep into the state of ground water quality in the district of Tiruvannamalai, Tamil Nadu, India, from a radiological and chemical toxicity perspective. Appl Water Sci 11, 83 (2021). https://doi.org/10.1007/s13201-021-01411-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01411-7