Abstract
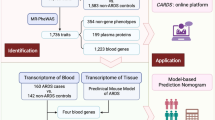
Acute aortic dissection (AAD) is a severe aortic injury disease, which is often life-threatening at the onset. However, its early prevention remains a challenge. Therefore, in the context of predictive, preventive, and personalized medicine (PPPM), it is particularly important to identify novel and powerful biomarkers. This study aimed to identify the key markers that may contribute to the predictive early risk of AAD and analyze their role in immune infiltration. Three datasets, including a total of 23 AAD and 20 healthy control aortic samples, were retrieved from the Gene Expression Omnibus (GEO) database, and a total of 519 differentially expressed genes (DEGs) were screened in the training set. Using the least absolute shrinkage and selection operator (LASSO) regression model and the random forest (RF) algorithm, FERMT1 (AUC = 0.886) and SGCD (AUC = 0.876) were identified as key markers of AAD. A novel AAD risk prediction model was constructed using an artificial neural network (ANN), and in the validation set, the AUC = 0.920. Immune infiltration analysis indicated differential gene expression in regulatory T cells, monocytes, γδ T cells, quiescent NK cells, and mast cells in the patients with AAD and the healthy controls. Correlation and ssGSEA analysis showed that two key markers’ expression in patients with AAD was correlated with many inflammatory mediators and pathways. In addition, the drug-gene interaction network identified motesanib and pyrazoloacridine as potential therapeutic agents for two key markers, which may provide personalized medical services for AAD patients. These findings highlight FERMT1 and SGCD as key biological targets for AAD and reveal the inflammation-related potential molecular mechanism of AAD, which is helpful for early risk prediction and targeted prevention of AAD. In conclusion, our study provides a new perspective for developing a PPPM method for managing AAD patients.











Similar content being viewed by others
Code availability
The code that supports the findings of this study is available from the corresponding author upon request.
References
Nienaber CA, Clough RE, Sakalihasan N, Suzuki T, Gibbs R, Mussa F, et al. Aortic dissection. Nat Rev Dis Primers. 2016;2:16053. https://doi.org/10.1038/nrdp.2016.53.
Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55–66. https://doi.org/10.1016/S0140-6736(08)60994-0.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897. https://doi.org/10.1001/jama.283.7.897.
Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. https://doi.org/10.1378/chest.117.5.1271.
Zhu Y, Lingala B, Baiocchi M, Tao JJ, Toro Arana V, Khoo JW, et al. Type A aortic dissection—experience over 5 decades. J Am Coll Cardiol. 2020;76:1703–13. https://doi.org/10.1016/j.jacc.2020.07.061.
Nienaber CA, Clough RE. Management of acute aortic dissection. The Lancet. 2015;385:800–11. https://doi.org/10.1016/S0140-6736(14)61005-9.
Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, et al. EPMA position paper in cancer: current overview and future perspectives. EPMA Journal. 2015;6:9. https://doi.org/10.1186/s13167-015-0030-6.
Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–60. https://doi.org/10.1161/CIRCULATIONAHA.117.031264.
Klompas M. Does this patient have an acute thoracic aortic dissection? 2000;11. https://doi.org/10.1001/jama.287.17.2262
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA Journal. 2016;7:23. https://doi.org/10.1186/s13167-016-0072-4.
Dalman RL, Wanhainen A, Mani K, Modarai B. Top 10 candidate aortic disease trials. J Intern Med. 2020;288:23–37. https://doi.org/10.1111/joim.13042.
Bossone E, Czerny M, Lerakis S, Rodríguez-Palomares J, Kukar N, Ranieri B, et al. Imaging and biomarkers in acute aortic syndromes: diagnostic and prognostic implications. Curr Probl Cardiol. 2021;46:100654. https://doi.org/10.1016/j.cpcardiol.2020.100654.
Nazerian P, Mueller C. Soeiro A de M, Leidel BA, Salvadeo SAT, Giachino F, et al Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes the ADvISED Prospective Multicenter Study. Circulation. 2018;137:250–8. https://doi.org/10.1161/CIRCULATIONAHA.117.029457.
Fletcher AJ, Syed MBJ, Aitman TJ, Newby DE, Walker NL. Inherited thoracic aortic disease: new insights and translational targets. Circulation. 2020;141:1570–87. https://doi.org/10.1161/CIRCULATIONAHA.119.043756.
Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33:26–35. https://doi.org/10.1093/eurheartj/ehx319.
Skotsimara G. Aortic wall inflammation in the pathogenesis, diagnosis and treatment of aortic aneurysms. :12. https://doi.org/10.1007/s10753-022-01626-z
Del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, et al. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42:622–9. https://doi.org/10.3109/07853890.2010.518156.
Cifani N, Proietta M, Tritapepe L, Di Gioia C, Ferri L, Taurino M, et al. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review. Ann Med. 2015;47:441–6. https://doi.org/10.3109/07853890.2015.1073346.
Stranneheim H, Wedell A. Exome and genome sequencing: a revolution for the discovery and diagnosis of monogenic disorders. J Intern Med. 2016;279:3–15. https://doi.org/10.1111/joim.12399.
Deo RC. Machine learning in medicine. Circulation. 2015;132:1920–30. https://doi.org/10.1161/CIRCULATIONAHA.115.001593.
Greener JG, Kandathil SM, Moffat L, Jones DT. A guide to machine learning for biologists. Nat Rev Mol Cell Biol. 2022;23:40–55. https://doi.org/10.1038/s41580-021-00407-0.
Buzdin A, Sorokin M, Garazha A, Glusker A, Aleshin A, Poddubskaya E, et al. RNA sequencing for research and diagnostics in clinical oncology. Semin Cancer Biol. 2020;60:311–23. https://doi.org/10.1016/j.semcancer.2019.07.010.
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The SVA package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3. https://doi.org/10.1093/bioinformatics/bts034.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43:e47–e47. https://doi.org/10.1093/nar/gkv007.
Carlson M. org.Mm.eg.db [Internet]. Bioconductor; 2017 [cited 2022 Apr 29]: https://bioconductor.org/packages/org.Mm.eg.dbhttps://doi.org/10.18129/B9.BIOC.ORG.MM.EG.DB.
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS A Journal of Integrative Biology. 2012;16:284–7. https://doi.org/10.1089/omi.2011.0118.
Tibshirani R. The Lasso method for variable selection in the Cox model. Statist Med. 1997;16:385–95. https://doi.org/10.1002/(SICI)1097-0258(19970228)16:4%3c385::AID-SIM380%3e3.0.CO;2-3.
Breiman L. No title found. Mach Learn. 2001;45:5–32. https://doi.org/10.1023/A:1010933404324.
Liaw A, Wiener M. Classification and regression by randomForest. 2002;2:5.
Moskowitz CS. Using free-response receiver operating characteristic curves to assess the accuracy of machine diagnosis of cancer. JAMA. 2017;318:2250. https://doi.org/10.1001/jama.2017.18686.
Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to machine learning, Neural Networks, and Deep Learning. Neural Networks. :12. https://doi.org/10.1167/tvst.9.2.14
Beck MW. NeuralNetTools : Visualization and analysis tools for neural networks. J Stat Soft [Internet]. 2018 [cited 2022 Apr 29];85. Available from: http://www.jstatsoft.org/v85/i11/
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. https://doi.org/10.1038/nmeth.3337.
Kim Y, Kang JW, Kang J, Kwon EJ, Ha M, Kim YK, et al. Novel deep learning-based survival prediction for oral cancer by analyzing tumor-infiltrating lymphocyte profiles through CIBERSORT. OncoImmunology. 2021;10:1904573. https://doi.org/10.1080/2162402X.2021.1904573.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–8. https://doi.org/10.1038/nprot.2008.73.
Chou K-C, Shen H-B. Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat Protoc. 2008;3:153–62. https://doi.org/10.1038/nprot.2007.494.
Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2009;NA-NA. https://doi.org/10.1002/jcc.21334.
W. Caldwell G, Yan Z, Lang W, A. Masucci J. The IC50 concept revisited. CTMC 2012;12:1282–90. https://doi.org/10.2174/156802612800672844.
Renard M. Clinical validity of genes for heritable thoracic aortic aneurysm and dissection. 2018;72:11. https://doi.org/10.1016/j.jacc.2018.04.089.
Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331–48. https://doi.org/10.1038/s41569-020-00472-6.
Reel PS, Reel S, Pearson E, Trucco E, Jefferson E. Using machine learning approaches for multi-omics data analysis: a review. Biotechnol Adv. 2021;49:107739. https://doi.org/10.1016/j.biotechadv.2021.107739.
Fernandez-Delgado M, Cernadas E, Barro S, Amorim D. Do we need hundreds of classifiers to solve real world classification problems? :49.
Zhang Y, Lin H, Yang Z, Wang J, Sun Y, Xu B, et al. Neural network-based approaches for biomedical relation classification: a review. J Biomed Inform. 2019;99:103294. https://doi.org/10.1016/j.jbi.2019.103294.
Rognoni E, Ruppert R, Fässler R. The kindlin family: functions, signaling properties and implications for human disease. J Cell Sci. 2016;129:17–27. https://doi.org/10.1242/jcs.161190.
Siegel DH, Ashton GHS, Penagos HG, Lee JV, Feiler HS, Wilhelmsen KC, et al. Loss of Kindlin-1, a human homolog of the Caenorhabditis elegans actin–extracellular-matrix linker protein UNC-112, causes Kindler syndrome. The American Journal of Human Genetics. 2003;73:174–87. https://doi.org/10.1086/376609.
Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115:4011–7. https://doi.org/10.1182/blood-2009-10-239269.
Qu H, Wen T, Pesch M, Aumailley M. Partial loss of epithelial phenotype in Kindlin-1–deficient keratinocytes. Am J Pathol. 2012;180:1581–92. https://doi.org/10.1016/j.ajpath.2012.01.005.
Rognoni E, Widmaier M, Jakobson M, Ruppert R, Ussar S, Katsougkri D, et al. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Nat Med. 2014;20:350–9. https://doi.org/10.1038/nm.3490.
Bauer R, Blain A, Greally E, Bushby K, Lochmüller H, Laval S, et al. Intolerance to β-blockade in a mouse model of δ-sarcoglycan-deficient muscular dystrophy cardiomyopathy. Eur J Heart Fail. 2010;12:1163–70. https://doi.org/10.1093/eurjhf/hfq129.
Isselbacher EM, Lino Cardenas CL, Lindsay ME. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation. 2016;133:2516–28. https://doi.org/10.1161/CIRCULATIONAHA.116.009762.
Heydemann A, McNally EM. Consequences of disrupting the dystrophin-sarcoglycan complex in Cardiac and Skeletal Myopathy. Trends Cardiovasc Med. 2007;17:55–9. https://doi.org/10.1016/j.tcm.2006.12.002.
He R, Guo D-C, Estrera AL, Safi HJ, Huynh TT, Yin Z, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671-678.e2. https://doi.org/10.1016/j.jtcvs.2005.09.018.
Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–58. https://doi.org/10.1016/j.immuni.2008.02.017.
Forrer A, Schoenrath F, Torzewski M, Schmid J, Franke UFW, Göbel N, et al. Novel blood biomarkers for a diagnostic workup of acute aortic dissection. Diagnostics. 2021;11:615. https://doi.org/10.3390/diagnostics11040615.
Li X, Liu D, Zhao L, Wang L, Li Y, Cho K, et al. Targeted depletion of monocyte/macrophage suppresses aortic dissection with the spatial regulation of MMP-9 in the aorta. Life Sci. 2020;254:116927. https://doi.org/10.1016/j.lfs.2019.116927.
Cifani N, Proietta M, Taurino M, Tritapepe L, Del Porto F. Monocyte Subsets, Stanford-A acute aortic dissection, and carotid artery stenosis: new evidences. J Immunol Res. 2019;2019:1–6. https://doi.org/10.1155/2019/9782594.
Tieu BC, Lee C, Sun H, LeJeune W, Recinos A, Ju X, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–51. https://doi.org/10.1172/JCI38308.
Tomida S, Aizawa K, Nishida N, Aoki H, Imai Y, Nagai R, et al. Indomethacin reduces rates of aortic dissection and rupture of the abdominal aorta by inhibiting monocyte/macrophage accumulation in a murine model. Sci Rep. 2019;9:10751. https://doi.org/10.1038/s41598-019-46673-z.
Padang R, Bagnall RD, Richmond DR, Bannon PG, Semsarian C. Rare non-synonymous variations in the transcriptional activation domains of GATA5 in bicuspid aortic valve disease. J Mol Cell Cardiol. 2012;53:277–81. https://doi.org/10.1016/j.yjmcc.2012.05.009.
Gao Y, Wang Z, Zhao J, Sun W, Guo J, Yang Z, et al. Involvement of B cells in the pathophysiology of β-aminopropionitrile-induced thoracic aortic dissection in mice. Exp Anim. 2019;68:331–9. https://doi.org/10.1538/expanim.18-0170.
Howard DPJ, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control. :12. https://doi.org/10.1161/CIRCULATIONAHA.112.000483
Pan L, Lin Z, Tang X, Tian J, Zheng Q, Jing J, et al. S-nitrosylation of plastin-3 exacerbates thoracic aortic dissection formation via endothelial barrier dysfunction. ATVB. 2020;40:175–88.
Kruser TJ, Wheeler DL, Armstrong EA, Iida M, Kozak KR, van der Kogel AJ, et al. Augmentation of radiation response by motesanib, a aultikinase inhibitor that targets vascular endothelial growth factor receptors. Clin Cancer Res. 2010;16:3639–47. https://doi.org/10.1161/ATVBAHA.119.313440.
Christianmd M, Trimblemdmph E. Salvage chemotherapy for epithelial ovarian carcinoma. Gynecol Oncol. 1994;55:S143–50. https://doi.org/10.1006/gyno.1994.1354.
Acknowledgements
We would like to thank the Gene Expression Omnibus (GEO) database for the precious data used for free in scientific research.
Funding
This work was supported by the Construction of key laboratories in Xinjiang Uygur Autonomous Region (NO.2019D04017) and the Tianshan cedar Project Fund of Xinjiang (2020XS13).
Author information
Authors and Affiliations
Contributions
Mierxiati: study design, data collection, statistical analysis, visualization, writing, and revised original draft. Wang Qi and Gulinazi revised the manuscript and performed the experiments. Ma Xiang led the study and provided scientific supervision. The final draft was verified by all authors before the submission.
Corresponding author
Ethics declarations
Ethics approval
Data retrieved from the GEO database was collected from patients who provided informed consent based on guidelines laid out by the GEO Ethics, Law, and Policy Group. The procedures used in this study adhere to the tenets of the Declaration of Helsinki and approval was obtained from the ethics committee of Xinjiang Medical University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ainiwan, M., Wang, Q., Yesitayi, G. et al. Identification of FERMT1 and SGCD as key marker in acute aortic dissection from the perspective of predictive, preventive, and personalized medicine. EPMA Journal 13, 597–614 (2022). https://doi.org/10.1007/s13167-022-00302-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-022-00302-4