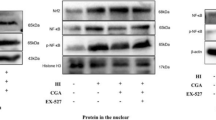
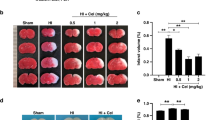
Abstract
Our previous studies have indicated that heliox preconditioning (HePC) may exert neuroprotective effects on neonatal hypoxic-ischemic encephalopathy (HIE). The present study was to investigate whether HePC alleviates neonatal HIE by inhibiting necroptosis and explore the potential mechanism. Seven-day-old rat pups were randomly divided into Sham group, HIE group, HIE + HePC group, HIE + Dantrolene (DAN) group, and HIE + Necrostatin-1 (Nec-1) group. HIE was induced by common carotid artery ligation and subsequent hypoxia exposure. The neurological function, brain injury, and molecular mechanism were evaluated by histological staining, neurobehavioral test, Western blotting, Ca2+, immunofluorescence staining, co-immunoprecipitation (Co-IP), and transmission electron microscopy (TEM). Results supported that the expression of necroptosis markers and p-RyR2 in the brain increased significantly after HIE. HePC, DAN, or Nec-1 was found to improve the neurological deficits after H/I and inhibit neuronal necroptosis. Interestingly, both HePC and DAN inhibited the increases in cytoplasmic Ca2+ and CaMK-II phosphorylation in the brain secondary to HIE, but Nec-1 failed to affect Ca2+. In conclusion, our results suggest HePC may alleviate cytoplasmic Ca2+ overload by regulating p-RyR2, which inhibits the necroptosis in the brain, exerting neuroprotective effects on HIE.








Similar content being viewed by others
Data Availability
The original contributions presented in the study are included in the article/supplementary materials; further inquiries can be directed to the corresponding authors.
References
Turlova E, Wong R, Xu B, Li F, Du L, Habbous S, Horgen FD, Fleig A, Feng ZP, Sun HS. TRPM7 mediates neuronal cell death upstream of calcium/calmodulin-dependent protein kinase II and calcineurin mechanism in neonatal hypoxic-ischemic brain injury. Transl Stroke Res. 2021;12(1):164–84.
Narayanamurthy R, Yang JJ, Yager JY, Unsworth LD. Drug delivery platforms for neonatal brain injury. J controlled release : official journal of the Controlled Release Society. 2021;330:765–87.
Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Human Dev. 2010;86(6):329–38.
Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatrics. 2015;169(4):397–403.
Dixon BJ, Reis C, Ho WM, Tang J, Zhang JH. Neuroprotective strategies after neonatal hypoxic ischemic encephalopathy. Intl J Mol Sci. 2015;16(9):22368–401.
Millar LJ, Shi L, Hoerder-Suabedissen A, Molnár Z. Neonatal hypoxia ischaemia: mechanisms, models, and therapeutic challenges. Front Cell Neurosci. 2017;11:78.
Al Mamun A, Yu H, Sharmeen R, et al. IRF5 signaling in phagocytes is detrimental to neonatal hypoxic ischemic encephalopathy. Transl Stroke Res. 2021;12:602–14.
Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10(4):372–82.
Jun-Long H, Yi L, Bao-Lian Z, Jia-Si L, Ning Z, Zhou-Heng Y, et al. Necroptosis signaling pathways in stroke: from mechanisms to therapies. Curr Neuropharmacol. 2018;16(9):1327–39.
Prempunpong C, Efanov I, Sant’Anna G. Serum calcium concentrations and incidence of hypocalcemia in infants with moderate or severe hypoxic-ischemic encephalopathy: effect of therapeutic hypothermia. Early Human Dev. 2015;91(9):535–40.
Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96(4):1261–96.
Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, et al. Endoplasmic reticulum stress in cerebral ischemia. Neurochem Intl. 2014;68:18–27.
Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128(5):893–904.
Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am College Cardiol. 2009;53(21):1993–2005.
Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem. 1996;67(6):2390–8.
Toussaint F, Charbel C, Blanchette A, Ledoux J. CaMKII regulates intracellular Ca2+ dynamics in native endothelial cells. Cell Calcium. 2015;58(3):275–85.
Nomura M, Ueno A, Saga K, Fukuzawa M, Kaneda Y. Accumulation of cytosolic calcium induces necroptotic cell death in human neuroblastoma. Cancer Res. 2014;74(4):1056–66.
Smit KF, Weber NC, Hollmann MW, Preckel B. Noble gases as cardioprotectants - translatability and mechanism. British J Pharmacol. 2015;172(8):2062–73.
Hyldegaard O, Jensen T. Effect of heliox, oxygen and air breathing on helium bubbles after heliox diving. Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society, Inc. 2007;34(2):107-22.
Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. The Journal of allergy and clinical immunology. 2007;120(5 Suppl):S94-138.
Pan Y, Zhang H, Acharya AB, Cruz-Flores S, Panneton WM. The effect of heliox treatment in a rat model of focal transient cerebral ischemia. Neurosci Lett. 2011;497(2):144–7.
David HN, Haelewyn B, Chazalviel L, Lecocq M, Degoulet M, Risso JJ, et al. Post-ischemic helium provides neuroprotection in rats subjected to middle cerebral artery occlusion-induced ischemia by producing hypothermia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29(6):1159–65.
Oei GT, Huhn R, Heinen A, Hollmann MW, Schlack WS, Preckel B, et al. Helium-induced cardioprotection of healthy and hypertensive rat myocardium in vivo. Eur J Pharmacol. 2012;684(1-3):125–31.
Zhang R, Zhang L, Manaenko A, Ye Z, Liu W, Sun X. Helium preconditioning protects mouse liver against ischemia and reperfusion injury through the PI3K/Akt pathway. J Hepatol. 2014;61(5):1048–55.
Pan Y, Zhang H, VanDeripe DR, Cruz-Flores S, Panneton WM. Heliox and oxygen reduce infarct volume in a rat model of focal ischemia. Exp Neurol. 2007;205(2):587–90.
Du L, Zhang R, Luo T, Nie M, Bi J. Effects of helium preconditioning on intestinal ischemia and reperfusion injury in rats. Shock (Augusta, Ga). 2015;44(4):365–70.
Li Y, Zhang P, Liu Y, Liu W, Yin N. Helium preconditioning protects the brain against hypoxia/ischemia injury via improving the neurovascular niche in a neonatal rat model. Behav Brain Res. 2016;314:165–72.
Li Y, Liu K, Kang ZM, Sun XJ, Liu WW, Mao YF. Helium preconditioning protects against neonatal hypoxia-ischemia via nitric oxide mediated up-regulation of antioxidases in a rat model. Behav Brain Res. 2016;300:31–7.
Heinen A, Huhn R, Smeele KM, Zuurbier CJ, Schlack W, Preckel B, et al. Helium-induced preconditioning in young and old rat heart: impact of mitochondrial Ca(2+) -sensitive potassium channel activation. Anesthesiology. 2008;109(5):830–6.
Pagel PS, Krolikowski JG, Amour J, Warltier DC, Weihrauch D. Morphine reduces the threshold of helium preconditioning against myocardial infarction: the role of opioid receptors in rabbits. J Cardiothor Vasc Anesth. 2009;23(5):619–24.
Haraschak JL, Langston VC, Wang R, Riggs C, Fellman C, Ross MK, et al. Pharmacokinetic evaluation of oral dantrolene in the dog. J Veterinary Pharmacol Ther. 2014;37(3):286–94.
Nakamura-Maruyama E, Miyamoto O, Okabe N, Himi N, Feng L, Narita K, et al. Ryanodine receptors contribute to the induction of ischemic tolerance. Brain Res Bull. 2016;122:45–53.
Zhou K, Shi L, Wang Z, Zhou J, Manaenko A, Reis C, et al. RIP1-RIP3-DRP1 pathway regulates NLRP3 inflammasome activation following subarachnoid hemorrhage. Exp Neurol. 2017;295:116–24.
Geng F, Yin H, Li Z, Li Q, He C, Wang Z, et al. Quantitative analysis of necrostatin-1, a necroptosis inhibitor by LC-MS/MS and the study of its pharmacokinetics and bioavailability. Biomed Pharmacother Biomed Pharmacother. 2017;95:1479–85.
Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–41.
Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55(2):158–63.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–6.
Ren Z, Zhang R, Li Y, Li Y, Yang Z, Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Intl J Mol Med. 2017;40(5):1444–56.
Liu Y, Xue F, Liu G, Shi X, Liu Y, Liu W, et al. Helium preconditioning attenuates hypoxia/ischemia-induced injury in the developing brain. Brain Res. 2011;1376:122–9.
Taherianfard M, Riyahi M, Razavi M, Bavandi Z, Eskandari Roozbahani N, Namavari MM. The cataleptic, asymmetric, analgesic, and brain biochemical effects of Parkinson’s disease can be affected by Toxoplasma gondii infection. BioMed Res Intl. 2020;2020:2546365.
Tassinari ID, Andrade MKG, da Rosa LA, Hoff MLM, Nunes RR, Vogt EL, et al. Lactate administration reduces brain injury and ameliorates behavioral outcomes following neonatal hypoxia-ischemia. Neuroscience. 2020;448:191–205.
Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104.
Mimura T, Dezawa M, Kanno H, Yamamoto I. Behavioral and histological evaluation of a focal cerebral infarction rat model transplanted with neurons induced from bone marrow stromal cells. J Neuropathol Exp Neurol. 2005;64(12):1108–17.
Fukunaga A, Uchida K, Hara K, Kuroshima Y, Kawase T. Differentiation and angiogenesis of central nervous system stem cells implanted with mesenchyme into ischemic rat brain. Cell Transplantation. 1999;8(4):435–41.
Gamdzyk M, Doycheva DM, Malaguit J, Enkhjargal B, Tang J, Zhang JH. Role of PPAR-β/δ/miR-17/TXNIP pathway in neuronal apoptosis after neonatal hypoxic-ischemic injury in rats. Neuropharmacology. 2018;140:150–61.
Zhang Y, Zhang X, Wei Q, Leng S, Li C, Han B, et al. Activation of Sigma-1 receptor enhanced pericyte survival via the interplay between apoptosis and autophagy: implications for blood-brain barrier integrity in stroke. Trans Stroke Res. 2020;11(2):267–87.
Mo ZT, Fang YQ, He YP, Zhang S. β-Asarone protects PC12 cells against OGD/R-induced injury via attenuating Beclin-1-dependent autophagy. Acta Pharmacologica Sinica. 2012;33(6):737–42.
Liu B, Wang D, Luo E, Hou J, Qiao Y, Yan G, et al. Role of TG2-mediated SERCA2 serotonylation on hypoxic pulmonary vein remodeling. Front Pharmacol. 2019;10:1611.
Dildy-Mayfield JE, Machu T, Leslie SW. Ethanol and voltage- or receptor-mediated increases in cytosolic Ca2+ in brain cells. Alcohol (Fayetteville, NY). 1992;9(1):63–9.
Mandal SK, Pendurthi UR, Rao LV. Tissue factor trafficking in fibroblasts: involvement of protease-activated receptor-mediated cell signaling. Blood. 2007;110(1):161–70.
Zhang R, Yu Y, Manaenko A, Bi H, Zhang N, Zhang L, et al. Effect of helium preconditioning on neurological decompression sickness in rats. Journal of applied physiology (Bethesda, Md : 1985). 2019;126(4):934-40.
Pagel PS, Krolikowski JG, Pratt PF Jr, Shim YH, Amour J, Warltier DC, et al. Inhibition of glycogen synthase kinase or the apoptotic protein p53 lowers the threshold of helium cardioprotection in vivo: the role of mitochondrial permeability transition. Anesthesia and Analgesia. 2008;107(3):769–75.
Pagel PS, Krolikowski JG. Transient metabolic alkalosis during early reperfusion abolishes helium preconditioning against myocardial infarction: restoration of cardioprotection by cyclosporin A in rabbits. Anesthesia and Analgesia. 2009;108(4):1076–82.
Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: mechanisms and relevance to disease. Ann Review Pathol. 2017;12:103–30.
Li SJ, Liu W, Wang JL, Zhang Y, Zhao DJ, Wang TJ, et al. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci. 2014;18(6):905–9.
Massaro AN, Wu YW, Bammler TK, Comstock B, Mathur A, McKinstry RC, et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2018;194:67–75.e1.
Huang J, Lu W, Doycheva DM, Gamdzyk M, Hu X, Liu R, et al. IRE1α inhibition attenuates neuronal pyroptosis via miR-125/NLRP1 pathway in a neonatal hypoxic-ischemic encephalopathy rat model. J Neuroinflamm. 2020;17(1):152.
Begum G, Kintner D, Liu Y, Cramer SW, Sun D. DHA inhibits ER Ca2+ release and ER stress in astrocytes following in vitro ischemia. J Neurochem. 2012;120(4):622–30.
Ramírez OA, Córdova A, Cerda M, Lobos P, Härtel S, Couve A, et al. Ryanodine receptor-mediated Ca(2+) release and atlastin-2 GTPase activity contribute to IP(3)-induced dendritic Ca(2+) signals in primary hippocampal neurons. Cell Calcium. 2021;96:102399.
Zheng Y, Shi G, Cai J, Yang J, Zhang Y, Gong Y, et al. Di-(2-ethyl hexyl) phthalate induces necroptosis in chicken cardiomyocytes by triggering calcium overload. J Hazard Mater. 2020;387:121696.
Qu Y, Tang J, Wang H, Li S, Zhao F, Zhang L, et al. RIPK3 interactions with MLKL and CaMKII mediate oligodendrocytes death in the developing brain. Cell Death Disease. 2017;8(2):e2629.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81873789) and the Medical-Engineering Cross Fund of Shanghai Jiao Tong University (No. YG2019GD03).
Author information
Authors and Affiliations
Contributions
WZ, JC, and XY made the idea and experimental design of this study. WZ, JC, WL, and YL performed most of the experiments. WZ and WL drafted the manuscript. WZ and YL approved the final version of the manuscript on behalf of all the authors. All authors did the critical revision and language modification of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All the experimental protocols were approved by the Ethics Committee of Shanghai Jiaotong University School of Medicine. This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary A
Experimental schematics. B Proposed Ca2+ -mediated signaling cascade following H/I. (PPTX 28462 kb)
Rights and permissions
About this article
Cite this article
Zhong, W., Cheng, J., Yang, X. et al. Heliox Preconditioning Exerts Neuroprotective Effects on Neonatal Ischemia/Hypoxia Injury by Inhibiting Necroptosis Induced by Ca2+ Elevation. Transl. Stroke Res. 14, 409–424 (2023). https://doi.org/10.1007/s12975-022-01021-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01021-8