Abstract
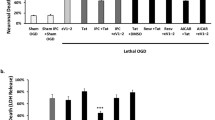
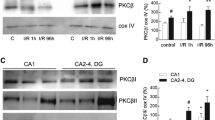
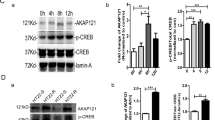
The preservation of mitochondrial function is a major protective strategy for cerebral ischemic injuries. Previously, our laboratory demonstrated that protein kinase C epsilon (PKCε) promotes the synthesis of mitochondrial nicotinamide adenine dinucleotide (NAD+). NAD+ along with its reducing equivalent, NADH, is an essential co-factor needed for energy production from glycolysis and oxidative phosphorylation. Yet, NAD+/NADH are impermeable to the inner mitochondrial membrane and their import into the mitochondria requires the activity of specific shuttles. The most important neuronal NAD+/NADH shuttle is the malate-aspartate shuttle (MAS). The MAS has been implicated in synaptic function and is potentially dysregulated during cerebral ischemia. The aim of this study was to determine if metabolic changes induced by PKCε preconditioning involved regulation of the MAS. Using primary neuronal cultures, we observed that the activation of PKCε enhanced mitochondrial respiration and glycolysis in vitro. Conversely, inhibition of the MAS resulted in decreased oxidative phosphorylation and glycolytic capacity. We further demonstrated that activation of PKCε increased the phosphorylation of key components of the MAS in rat brain synaptosomal fractions. Additionally, PKCε increased the enzyme activity of glutamic oxaloacetic transaminase 2 (GOT2), an effect that was dependent on the import of PKCε into the mitochondria and phosphorylation of GOT2. Furthermore, PKCε activation was able to rescue decreased GOT2 activity induced by ischemia. These findings reveal novel protective targets and mechanisms against ischemic injury, which involves PKCε-mediated phosphorylation and activation of GOT2 in the MAS.







Similar content being viewed by others
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–66. https://doi.org/10.1161/CIR.0000000000000659.
Jackson CW, Escobar I, Xu J, Perez-Pinzon MA. Effects of ischemic preconditioning on mitochondrial and metabolic neruoprotection: 5′ adenosine monophosphate-activated protein kinase and sirtuins. Brain Circ. 2018;4(2):54–61. https://doi.org/10.4103/bc.bc_7_18.
Kristin V. Ischemic conditioning in organ transplantation. Cond Med. 2018;1(4):212–9.
Cuomo O, Vinciguerra A, Cepparulo P, Anzilotti S, Brancaccio P, Formisano L, et al. Differences and similarities in neuroprotective molecular pathways activated by distinct preconditioning inducers. Cond Med. 2018;1(4):187–203.
Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23(2):384–91.
Li J, Niu C, Han S, Zu P, Li H, Xu Q, et al. Identification of protein kinase C isoforms involved in cerebral hypoxic preconditioning of mice. Brain Res. 2005;1060(1–2):62–72. https://doi.org/10.1016/j.brainres.2005.08.047.
Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28(16):4172–82. https://doi.org/10.1523/JNEUROSCI.5471-07.2008.
Selvaraji S, Poh L, Natarajan V, Mallilankaraman K, Arumugam TV. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond Med. 2019;2(1):30–9.
Bastian C, Politano S, Day J, McCray A, Brunet S, Baltan S. Mitochondrial dynamics and preconditioning in white matter. Cond Med. 2018;1(2):64–72.
Owens K, Park JH, Schuh R, Kristian T. Mitochondrial dysfunction and NAD(+) metabolism alterations in the pathophysiology of acute brain injury. Transl Stroke Res. 2013;4(6):618–34. https://doi.org/10.1007/s12975-013-0278-x.
Morris-Blanco KC, Cohan CH, Neumann JT, Sick TJ, Perez-Pinzon MA. Protein kinase C epsilon regulates mitochondrial pools of Nampt and NAD following resveratrol and ischemic preconditioning in the rat cortex. J Cereb Blood Flow Metab. 2014;34(6):1024–32. https://doi.org/10.1038/jcbfm.2014.51.
Khoury N, Koronowski KB, Young JI, Perez-Pinzon MA. The NAD(+)-dependent family of sirtuins in cerebral ischemia and preconditioning. Antioxid Redox Signal. 2018;28(8):691–710. https://doi.org/10.1089/ars.2017.7258.
Lehninger AL. Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J Biol Chem. 1951;190(1):345–59.
McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71(4):399–407. https://doi.org/10.1016/j.bcp.2005.10.011.
Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134(12):883–94. https://doi.org/10.1161/CIRCULATIONAHA.116.022495.
Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, et al. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci. 2013;33(35):13957–71, 71a. https://doi.org/10.1523/JNEUROSCI.0929-13.2013.
Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, et al. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem. 2006;281(2):1039–47. https://doi.org/10.1074/jbc.M507270200.
Koronowski KB, Khoury N, Saul I, Loris ZB, Cohan CH, Stradecki-Cohan HM, et al. Neuronal SIRT1 (silent information regulator 2 homologue 1) regulates glycolysis and mediates resveratrol-induced ischemic tolerance. Stroke. 2017;48(11):3117–25. https://doi.org/10.1161/STROKEAHA.117.018562.
Khoury N, Xu J, Stegelmann SD, Jackson CW, Koronowski KB, Dave KR, et al. Resveratrol preconditioning induces genomic and metabolic adaptations within the long-term window of cerebral ischemic tolerance leading to bioenergetic efficiency. Mol Neurobiol. 2018;56:4549–65. https://doi.org/10.1007/s12035-018-1380-6.
Koronowski KB, Khoury N, Morris-Blanco KC, Stradecki-Cohan HM, Garrett TJ, Perez-Pinzon MA. Metabolomics based identification of SIRT5 and protein kinase C epsilon regulated pathways in brain. Front Neurosci. 2018;12:32. https://doi.org/10.3389/fnins.2018.00032.
Dimauro I, Pearson T, Caporossi D, Jackson MJ. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes. 2012;5:513. https://doi.org/10.1186/1756-0500-5-513.
Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70(2):222–30. https://doi.org/10.1016/j.cardiores.2006.02.015.
Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36(12):2781–90. https://doi.org/10.1161/01.STR.0000189996.71237.f7.
Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI, et al. Proteomic and metabolomic analysis of cardioprotection: interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J Mol Cell Cardiol. 2009;46(2):268–77. https://doi.org/10.1016/j.yjmcc.2008.10.008.
Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88(1):83–92. https://doi.org/10.1093/cvr/cvq154.
Thompson JW, Dave KR, Saul I, Narayanan SV, Perez-Pinzon MA. Epsilon PKC increases brain mitochondrial SIRT1 protein levels via heat shock protein 90 following ischemic preconditioning in rats. PLoS One. 2013;8(9):e75753. https://doi.org/10.1371/journal.pone.0075753.
Bunger R, Glanert S, Sommer O, Gerlach E. Inhibition by (aminooxy)acetate of the malate-aspartate cycle in the isolated working Guinea pig heart. Hoppe Seylers Z Physiol Chem. 1980;361(6):907–14.
Kauppinen RA, Sihra TS, Nicholls DG. Aminooxyacetic acid inhibits the malate-aspartate shuttle in isolated nerve terminals and prevents the mitochondria from utilizing glycolytic substrates. Biochim Biophys Acta. 1987;930(2):173–8.
Kim EJ, Raval AP, Hirsch N, Perez-Pinzon MA. Ischemic preconditioning mediates cyclooxygenase-2 expression via nuclear factor-kappa B activation in mixed cortical neuronal cultures. Transl Stroke Res. 2010;1(1):40–7.
Lange-Asschenfeldt C, Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24(6):636–45. https://doi.org/10.1097/01.WCB.0000121235.42748.BF.
Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90(4):390–7. https://doi.org/10.1161/01.res.0000012702.90501.8d.
Jung YS, Jung YS, Kim MY, Kim E. Identification of caspase-independent PKCepsilon-JNK/p38 MAPK signaling module in response to metabolic inhibition in H9c2 cells. Jpn J Physiol. 2004;54(1):23–9.
Ping P, Zhang J, Huang S, Cao X, Tang XL, Li RC, et al. PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Phys. 1999;277(5):H1771–85. https://doi.org/10.1152/ajpheart.1999.277.5.H1771.
Ping P, Zhang J, Zheng YT, Li RC, Dawn B, Tang XL, et al. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res. 1999;85(6):542–50. https://doi.org/10.1161/01.res.85.6.542.
Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC, et al. Formation of protein kinase C(epsilon)-Lck signaling modules confers cardioprotection. J Clin Invest. 2002;109(4):499–507. https://doi.org/10.1172/JCI13200.
Bae ON, Rajanikant K, Min J, Smith J, Baek SH, Serfozo K, et al. Lymphocyte cell kinase activation mediates neuroprotection during ischemic preconditioning. J Neurosci. 2012;32(21):7278–86. https://doi.org/10.1523/JNEUROSCI.6273-11.2012.
Juaristi I, Garcia-Martin ML, Rodrigues TB, Satrustegui J, Llorente-Folch I, Pardo B. ARALAR/AGC1 deficiency, a neurodevelopmental disorder with severe impairment of neuronal mitochondrial respiration, does not produce a primary increase in brain lactate. J Neurochem. 2017;142(1):132–9. https://doi.org/10.1111/jnc.14047.
Wang J, Bright R, Mochly-Rosen D, Giffard RG. Cell-specific role for epsilon- and betaI-protein kinase C isozymes in protecting cortical neurons and astrocytes from ischemia-like injury. Neuropharmacology. 2004;47(1):136–45. https://doi.org/10.1016/j.neuropharm.2004.03.009.
Mali Y, Zisapel N. VEGF up-regulation by G93A superoxide dismutase and the role of malate-aspartate shuttle inhibition. Neurobiol Dis. 2010;37(3):673–81. https://doi.org/10.1016/j.nbd.2009.12.005.
McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci. 1993;15(3–5):320–9. https://doi.org/10.1159/000111351.
Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–5. https://doi.org/10.1038/nature13909.
Stottrup NB, Lofgren B, Birkler RD, Nielsen JM, Wang L, Caldarone CA, et al. Inhibition of the malate-aspartate shuttle by pre-ischaemic aminooxyacetate loading of the heart induces cardioprotection. Cardiovasc Res. 2010;88(2):257–66. https://doi.org/10.1093/cvr/cvq205.
Dalgas C, Povlsen JA, Lofgren B, Erichsen SB, Botker HE. Effects of fatty acids on cardioprotection by pre-ischaemic inhibition of the malate-aspartate shuttle. Clin Exp Pharmacol Physiol. 2012;39(10):878–85. https://doi.org/10.1111/j.1440-1681.2012.05749.x.
Mitchell M, Cashman KS, Gardner DK, Thompson JG, Lane M. Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol Reprod. 2009;80(2):295–301. https://doi.org/10.1095/biolreprod.108.069864.
Datta A, Akatsu H, Heese K, Sze SK. Quantitative clinical proteomic study of autopsied human infarcted brain specimens to elucidate the deregulated pathways in ischemic stroke pathology. J Proteome. 2013;91:556–68. https://doi.org/10.1016/j.jprot.2013.08.017.
Rink C, Gnyawali S, Stewart R, Teplitsky S, Harris H, Roy S, et al. Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. FASEB J. 2017;31(4):1709–18. https://doi.org/10.1096/fj.201601033R.
Campos F, Sobrino T, Ramos-Cabrer P, Argibay B, Agulla J, Perez-Mato M, et al. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab. 2011;31(6):1378–86. https://doi.org/10.1038/jcbfm.2011.3.
Lofgren B, Povlsen JA, Rasmussen LE, Stottrup NB, Solskov L, Krarup PM, et al. Amino acid transamination is crucial for ischaemic cardioprotection in normal and preconditioned isolated rat hearts--focus on L-glutamate. Exp Physiol. 2010;95(1):140–52. https://doi.org/10.1113/expphysiol.2009.049452.
Della-Morte D, Raval AP, Dave KR, Lin HW, Perez-Pinzon MA. Post-ischemic activation of protein kinase C epsilon protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci Lett. 2011;487(2):158–62. https://doi.org/10.1016/j.neulet.2010.10.013.
Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, Perez-Pinzon MA. Protein kinase C epsilon promotes cerebral ischemic tolerance via modulation of mitochondrial Sirt5. Sci Rep. 2016;6:29790. https://doi.org/10.1038/srep29790.
Kumar V, Weng YC, Wu YC, Huang YT, Liu TH, Kristian T, et al. Genetic inhibition of PKCepsilon attenuates neurodegeneration after global cerebral ischemia in male mice. J Neurosci Res. 2019;97(4):444–55. https://doi.org/10.1002/jnr.24362.
Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366–71. https://doi.org/10.1073/pnas.0910280107.
Endo S, Ishiguro S, Tamai M. Possible mechanism for the decrease of mitochondrial aspartate aminotransferase activity in ischemic and hypoxic rat retinas. Biochim Biophys Acta. 1999;1450(3):385–96.
Hinkelbein J, Feldmann RE Jr, Kalenka A. Time-dependent alterations of cerebral proteins following short-term normobaric hyperoxia. Mol Cell Biochem. 2010;339(1–2):9–21. https://doi.org/10.1007/s11010-009-0365-1.
Allen EL, Ulanet DB, Pirman D, Mahoney CE, Coco J, Si Y, et al. Differential aspartate usage identifies a subset of cancer cells particularly dependent on OGDH. Cell Rep. 2016;17(3):876–90. https://doi.org/10.1016/j.celrep.2016.09.052.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–5. https://doi.org/10.1038/nature12040.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–51. https://doi.org/10.1016/j.cell.2015.07.016.
Fiermonte G, Walker JE, Palmieri F. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem J. 1993;294(Pt 1):293–9. https://doi.org/10.1042/bj2940293.
Monne M, Miniero DV, Iacobazzi V, Bisaccia F, Fiermonte G. The mitochondrial oxoglutarate carrier: from identification to mechanism. J Bioenerg Biomembr. 2013;45(1–2):1–13. https://doi.org/10.1007/s10863-012-9475-7.
Zhao JJ, Xiao H, Zhao WB, Zhang XP, Xiang Y, Ye ZJ, et al. Remote ischemic postconditioning for ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Chin Med J. 2018;131(8):956–65. https://doi.org/10.4103/0366-6999.229892.
Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, Randomized Controlled Trial. Circulation. 2017;135(14):1325–35. https://doi.org/10.1161/CIRCULATIONAHA.116.024807.
Acknowledgements
We would like to thank Clemer Abad for his assistance with the real-time PCR experiments.
Funding
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants NS45676, NS097658, and NS34773 (to M.A.P.P.), and the American Heart Association (AHA) predoctoral award 19PRE34400074 (to J.X.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed. Animal usage and experimentation were approved by the Institutional Animal Care and Use Committee at the University of Miami and was in accordance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, J., Khoury, N., Jackson, C.W. et al. Ischemic Neuroprotectant PKCε Restores Mitochondrial Glutamate Oxaloacetate Transaminase in the Neuronal NADH Shuttle after Ischemic Injury. Transl. Stroke Res. 11, 418–432 (2020). https://doi.org/10.1007/s12975-019-00729-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00729-4