Abstract
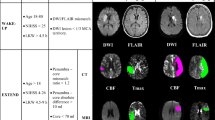
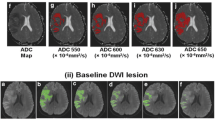
While several MRI parameters are used to assess tissue perfusion during hyperacute stroke, it is unclear which is optimal for measuring clinically relevant reperfusion. We directly compared mean transit time (MTT) prolongation (MTTp), time-to-peak (TTP), and time-to-maximum (Tmax) to determine which best predicted neurological improvement and tissue salvage following early reperfusion. Acute ischemic stroke patients underwent three MRIs: <4.5 h (tp1), at 6 h (tp2), and at 1 month after onset. Perfusion deficits at tp1 and tp2 were defined by MTTp, TTP, or Tmax beyond four commonly used thresholds. Percent reperfusion (%Reperf) was calculated for each parameter and threshold. Regression analysis was used to fit %Reperf for each parameter and threshold as a predictor of neurological improvement [defined as admission National Institutes of Health Stroke Scale (NIHSS)–1 month NIHSS (∆NIHSS)] after adjusting for baseline clinical variables. Volume of reperfusion, for each parameter and threshold, was correlated with tissue salvage, defined as tp1 perfusion deficit volume–final infarct volume. Fifty patients were scanned at 2.7 and 6.2 h after stroke onset. %Reperf predicted ∆NIHSS for all MTTp thresholds, for Tmax >6 s and >8 s, but for no TTP thresholds. Tissue salvage significantly correlated with reperfusion for all MTTp thresholds and with Tmax >6 s, while there was no correlation with any TTP threshold. Among all parameters, reperfusion defined by MTTp was most strongly associated with ∆NIHSS (MTTp >3 s, P = 0.0002) and tissue salvage (MTTp >3 s and 4 s, P < 0.0001). MTT-defined reperfusion was the best predictor of neurological improvement and tissue salvage in hyperacute ischemic stroke.


Similar content being viewed by others
References
Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12(6):723–5.
Ostergaard L. Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging. 2005;22(6):710–7.
Ostergaard L et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn Reson Med. 1996;36(5):726–36.
Davis SM et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309.
Hacke W et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–50.
Parsons M et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366(12):1099–107.
Butcher KS et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36(6):1153–9.
Schellinger PD et al. Stroke magnetic resonance imaging within 6 h after onset of hyperacute cerebral ischemia. Ann Neurol. 2001;49(4):460–9.
Chalela JA et al. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55(1):105–12.
Mishra NK et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke. 2011;41(1):e25–33.
Furlan A et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003–11.
Khatri P et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73(13):1066–72.
Wu O et al. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50(1):164–74.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56.
Shih LC et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34(6):1425–30.
Sobesky J et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35(12):2843–7.
Shah MK et al. Quantitative cerebral MR perfusion imaging: preliminary results in stroke. J Magn Reson Imaging. 2010;32(4):796–802.
Counsell C et al. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke. 2002;33(4):1041–7.
Adams Jr HP et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53(1):126–31.
Hacke W et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-h window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36(1):66–73.
Kane I et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38(12):3158–64.
Zaro-Weber O et al. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41(12):2817–21.
Galinovic I et al. Search for a map and threshold in perfusion MRI to accurately predict tissue fate: a protocol for assessing lesion growth in patients with persistent vessel occlusion. Cerebrovasc Dis. 2011;32(2):186–93.
Olivot JM et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40(2):469–75.
Albers GW et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–17.
Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–73.
Soares BP et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010;41(1):e34–40.
Yamada K et al. Magnetic resonance perfusion-weighted imaging of acute cerebral infarction: effect of the calculation methods and underlying vasculopathy. Stroke. 2002;33(1):87–94.
Calamante F et al. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke. 2010;41(6):1169–74.
Sources of Funding
This study was supported by grants from National Institutes of Health NIH 5P50NS055977 (to JML) and K23 NS069807 (to AF) and from Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Compliance with Ethics Requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
None of the authors have any financial relationships with the National Institutes of Health who funded this research and all the authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Andria L. Ford and Hongyu An contributed equally to the manuscript
Rights and permissions
About this article
Cite this article
Ford, A.L., An, H., Kong, L. et al. Clinically Relevant Reperfusion in Acute Ischemic Stroke: MTT Performs Better than Tmax and TTP. Transl. Stroke Res. 5, 415–421 (2014). https://doi.org/10.1007/s12975-014-0325-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-014-0325-2