Abstract
Purpose of Review
Young women represent a minority of breast cancer patients for which fertility, family planning, and pregnancy represent unique vulnerabilities. This review intends to discuss recent published evidence regarding treatment-related infertility, fertility counseling, and preservation.
Recent Findings
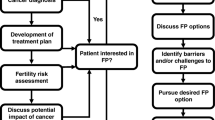
Fertility concerns are common among young women with breast cancer and may negatively affect treatment decisions. Data is available to aid providers in approximating odds of post-treatment amenorrhea and infertility. Multiple fertility preservation techniques are available. While embryo preservation is most commonly used, recent guidelines endorse oocyte preservation and support for ovarian tissue cryopreservation is increasing. Most recently, the contribution of ovarian suppression during chemotherapy to ovarian function preservation has been established. Germline BRCA mutations may impact fertility potential and challenge fertility preservation and preimplantation genetic testing should be discussed with this subset.
Summary
Fertility counseling and preservation have become an integral part of the multidisciplinary care for breast cancer at diagnosis and throughout survivorship. Efforts to further individualize recommendations are necessary.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. https://doi.org/10.1002/ijc.31937.
International Agency for Research on Cancer. Estimated number of new cases in 2018, worldwide, females, ages 0-44. Cancer Today. 2018. https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=8&nb_items=5&group_cancer=1&include_nmsc=1&include_nmsc_other=1. Accessed 10 Jan 2020.
Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast (Edinburgh, Scotland). 2017;35:203–17. https://doi.org/10.1016/j.breast.2017.07.017. Most recent consensus guidelines for the management of breast cancer in young women, highlighting unique aspects of screening, diagnosis, treatment and supportive care.
Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2017;110(4):426–9. https://doi.org/10.1093/jnci/djx206. This retrospective multicenter case-control study with a median follow-up of 7.2 years after pregnancy provides important reassurance regarding the long term safety of pregnancy after breast cancer, in particular for women with ER positive cancers.
OECD Family Database—OECD. http://www.oecd.org/els/family/database.htm. Accessed September 10, 2019.
Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. Journal of Clinical Oncology. 2004;22(20):4174–83. https://doi.org/10.1200/JCO.2004.01.159. This was a large early study highlighting the significance of fertility concerns among young breast cancer patients, their impact on treatment decisions and the need for better communication regarding these issues.
Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–6. https://doi.org/10.1200/JCO.2013.52.8877.
Ruggeri M, Pagan E, Bagnardi V, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European centers. Breast. 2019;47:85–92. https://doi.org/10.1016/j.breast.2019.07.001.
Thewes B, Meiser B, Rickard J, Friedlander M. The fertility- and menopause-related information needs of younger women with a diagnosis of breast cancer: a qualitative study. Psychooncology. 2003;12(5):500–11. https://doi.org/10.1002/pon.685.
Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. https://doi.org/10.1093/jnci/djr541.
Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107(10). doi:https://doi.org/10.1093/jnci/djv202
Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12(20):2333–44. https://doi.org/10.2217/fon-2016-0176.
Fertility and ageing. Hum Reprod Update. 2005;11(3):261–276. doi:https://doi.org/10.1093/humupd/dmi006
Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–403. https://doi.org/10.1093/humrep/17.5.1399.
Poorvu PD, Frazier AL, Feraco AM, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectrum. 2019;3(1):pkz008. https://doi.org/10.1093/jncics/pkz008. An encompassing review of infertility rates associated with various specific malignancies and modern therapies. An accurate understanding of risk is critical in counseling patients on fertility preservation.
Jacobson MH, Mertens AC, Spencer JB, Manatunga AK, Howards PP. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil Steril. 2016;105(3):765–772.e4. https://doi.org/10.1016/j.fertnstert.2015.11.020.
Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94(2):638–44. https://doi.org/10.1016/j.fertnstert.2009.03.045.
Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43(11):1646–53. https://doi.org/10.1016/j.ejca.2007.04.006.
Letourneau JM, Ebbel EE, Katz PP, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118(7):1933–9. https://doi.org/10.1002/cncr.26403.
Krekow LK, Hellerstedt BA, Collea RP, et al. Incidence and predictive factors for recovery of ovarian function in amenorrheic women in their 40s treated with letrozole. J Clin Oncol. 2016;34(14):1594–600. https://doi.org/10.1200/JCO.2015.62.2985.
Zavos A, Valachis A. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol. 2016;55(6):664–70. https://doi.org/10.3109/0284186X.2016.1155738.
Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. JCO. 2018;36(19):1981–90. https://doi.org/10.1200/JCO.2018.78.0858. A patient level metanalysis of five trials including premenopausal breast cancer patients providing quality evidence to support the use of ovarian suppression during chemotherapy to prevent premature ovarian insufficiency and potentially improve fertility outcomes.
Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. https://doi.org/10.1016/j.fertnstert.2010.04.006.
Wong QHY, Anderson RA. The role of antimullerian hormone in assessing ovarian damage from chemotherapy, radiotherapy and surgery. Current Opinion in Endocrinology, Diabetes and Obesity. 2018;25(6):391. https://doi.org/10.1097/MED.0000000000000447.
Phillips K-A, Collins IM, Milne RL, et al. Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod. 2016;31(5):1126–32. https://doi.org/10.1093/humrep/dew044.
Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–4. https://doi.org/10.1200/JCO.2009.24.2057.
Finch A, Valentini A, Greenblatt E, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99(6):1724–8. https://doi.org/10.1016/j.fertnstert.2013.01.109.
Ben-Aharon I, Levi M, Margel D, et al. Premature ovarian aging in BRCA carriers: a prototype of systemic precocious aging? Oncotarget. 2018;9(22):15931–41. https://doi.org/10.18632/oncotarget.24638.
Valentini A, Finch A, Lubinski J, et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2013;31(31):3914–9. https://doi.org/10.1200/JCO.2012.47.7893.
Lambertini M, Olympios N, Lequesne J, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-Müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00575.
Oktay K, Bedoschi G, Goldfarb SB, et al. Abstract PD6-06: impact of BRCA mutations on chemotherapy-induced loss of ovarian reserve: a prospective longitudinal study. Cancer Res. 2019;79(4 Supplement):PD6-06-PD6-06. https://doi.org/10.1158/1538-7445.SABCS18-PD6-06.
Sukumvanich P, Case LD, Zee KV, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment. Cancer. 2010;116(13):3102–11. https://doi.org/10.1002/cncr.25106.
Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116(4):791–8. https://doi.org/10.1002/cncr.24835.
Swain SM, Land SR, Ritter MW, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113(2):315–20. https://doi.org/10.1007/s10549-008-9937-0.
Treatment-related amenorrhea among young women one year following diagnosis of early-stage breast cancer. | Journal of Clinical Oncology. https://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.9523. Accessed November 3, 2019.
Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst. 2019;111(1):86–94. https://doi.org/10.1093/jnci/djy094.
Ruddy KJ, Guo H, Barry W, et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res Treat. 2015;151(3):589–96. https://doi.org/10.1007/s10549-015-3426-z.
Ganz PA, Land SR, Geyer CE, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29(9):1110–6. https://doi.org/10.1200/JCO.2010.29.7689. This large prospective study provided important information on the risk of amenorrhea associated with Anthracycline and taxane based chemotherapy protocols commonly used in the treatment of breast cancer.
Catlin NR, Bowman CJ, Engel SM, et al. Reproductive and developmental toxicity assessment of palbociclib, a CDK4/6 inhibitor, in Sprague-Dawley rats and New Zealand white rabbits. Reprod Toxicol. 2019;88:76–84. https://doi.org/10.1016/j.reprotox.2019.07.016.
Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. JCO. 2019. https://doi.org/10.1200/JCO.19.01304.
Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. JCO. 2006;24(7):1045–51. https://doi.org/10.1200/JCO.2005.03.3969.
Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. JCO. 2018;36(19):1994–2001. https://doi.org/10.1200/JCO.2018.78.1914.
Peccatori FA, Azim HA, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl_6):vi160–70. https://doi.org/10.1093/annonc/mdt199.
Goossens J, Delbaere I, Van Lancker A, Beeckman D, Verhaeghe S, Van Hecke A. Cancer patients’ and professional caregivers’ needs, preferences and factors associated with receiving and providing fertility-related information: a mixed-methods systematic review. Int J Nurs Stud. 2014;51(2):300–19. https://doi.org/10.1016/j.ijnurstu.2013.06.015.
Quinn GP, Vadaparampil ST, Lee J-H, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. JCO. 2009;27(35):5952–7. https://doi.org/10.1200/JCO.2009.23.0250.
Kim J, Mersereau JE, Su HI, Whitcomb BW, Malcarne VL, Gorman JR. Young female cancer survivors’ use of fertility care after completing cancer treatment. Support Care Cancer. 2016;24(7):3191–9. https://doi.org/10.1007/s00520-016-3138-x.
Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: a systematic review. Deshpande-2015-Cancer-Wiley Online Library. https://onlinelibrary.wiley.com/doi/full/10.1002/cncr.29637. Accessed September 26, 2019.
Logan S, Anazodo A. The psychological importance of fertility preservation counseling and support for cancer patients. Acta Obstet Gynecol Scand. 2019;98(5):583–97. https://doi.org/10.1111/aogs.13562.
National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Published January 18, 2019.
Chan JL, Johnson LN, Sammel MD, et al. Reproductive decision-making in women with BRCA1/2 mutations. J Genet Couns. 2017;26(3):594–603. https://doi.org/10.1007/s10897-016-0035-x.
Lambertini M, Goldrat O, Ferreira AR, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. 2018;29(1):237–43. https://doi.org/10.1093/annonc/mdx639.
Peccatori FA, Mangili G, Bergamini A, et al. Fertility preservation in women harboring deleterious BRCA mutations: ready for prime time? Hum Reprod. 2018;33(2):181–7. https://doi.org/10.1093/humrep/dex356.
Schover LR. Motivation for parenthood after cancer: a review. J Natl Cancer Inst Monogr. 2005;2005(34):2–5. https://doi.org/10.1093/jncimonographs/lgi010.
Lambertini M, Di Maio M, Pagani O, et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. 2018;42:41–9. https://doi.org/10.1016/j.breast.2018.08.099.
Azim HA, Santoro L, Pavlidis N, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47(1):74–83. https://doi.org/10.1016/j.ejca.2010.09.007.
Lambertini M, Ameye L, Hamy A-S, et al. Safety of pregnancy following breast cancer (BC) in patients (pts) carrying a BRCA mutation (mBRCA): results of an international cohort study. JCO. 2019;37(15_suppl):11506. https://doi.org/10.1200/JCO.2019.37.15_suppl.11506.
Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–46. https://doi.org/10.1200/JCO.1996.14.10.2738.
Gallos ID, Eapen A, Price MJ, et al. Controlled ovarian stimulation protocols for assisted reproduction: a network meta-analysis. Cochrane Database Syst Rev. 2017;3. https://doi.org/10.1002/14651858.CD012586.
Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–80. https://doi.org/10.1016/j.fertnstert.2013.07.1992.
Oktay K, Türkçüoğlu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod BioMed Online. 2010;20(6):783–8. https://doi.org/10.1016/j.rbmo.2010.03.004.
von Wolff M, Germeyer A, Liebenthron J, Korell M, Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: fertility preservation techniques. Arch Gynecol Obstet. 2018;297(1):257–67. https://doi.org/10.1007/s00404-017-4595-2.
Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–53. https://doi.org/10.1200/JCO.2005.05.037. Early study showing the feasibility of controlled ovarian stimulation with Tamoxifen and Letrozole and efficacy of the latter in reducing peak estrogen levels. More recent studies also support the safety of this approach which is now commonly used in fertility preservation in the setting of early breast cancer.
Meirow D, Raanani H, Maman E, et al. Tamoxifen co-administration during controlled ovarian hyperstimulation for in vitro fertilization in breast cancer patients increases the safety of fertility-preservation treatment strategies. Fertil Steril. 2014;102(2):488–495.e3. https://doi.org/10.1016/j.fertnstert.2014.05.017.
Rodgers RJ, Reid GD, Koch J, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod. 2017;32(5):1033–45. https://doi.org/10.1093/humrep/dex027.
Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101(4):1364–71. https://doi.org/10.1210/jc.2015-3878.
Kim J, Oktay K, Gracia C, Lee S, Morse C, Mersereau JE. Which patients pursue fertility preservation treatments? A multi-center analysis of the predictors of fertility preservation in women with breast cancer. Fertil Steril. 2012;97(3):671–6. https://doi.org/10.1016/j.fertnstert.2011.12.008.
Hershlag A, Mullin C, Bristow SL. Is fertility preservation feasible and safe with neoadjuvant therapy for breast cancer? J Glob Oncol. 2018. https://doi.org/10.1200/JGO.17.00213.
Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. JCO. 2013;31(19):2500–10. https://doi.org/10.1200/JCO.2013.49.2678.
Letourneau JM, Sinha N, Wald K, et al. Random start ovarian stimulation for fertility preservation appears unlikely to delay initiation of neoadjuvant chemotherapy for breast cancer. Hum Reprod. 2017;32(10):2123–9. https://doi.org/10.1093/humrep/dex276.
Chien AJ, Chambers J, Mcauley F, et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II–III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat. 2017;165(1):151–9. https://doi.org/10.1007/s10549-017-4288-3.
Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–36. https://doi.org/10.1056/NEJMoa1705334.
Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33(22):2424–9. https://doi.org/10.1200/JCO.2014.59.3723.
Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–55. https://doi.org/10.1093/humupd/dmw038.
Son W-Y, Henderson S, Cohen Y, Dahan M, Buckett W. Immature oocyte for fertility preservation. Front Endocrinol. 2019. https://doi.org/10.3389/fendo.2019.00464.
Creux H, Monnier P, Son W-Y, Buckett W. Thirteen years’ experience in fertility preservation for cancer patients after in vitro fertilization and in vitro maturation treatments. J Assist Reprod Genet. 2018;35(4):583–92. https://doi.org/10.1007/s10815-018-1138-0.
Fadini R, Mignini Renzini M, Dal Canto M, et al. Oocyte in vitro maturation in normo-ovulatory women. Fertil Steril. 2013;99(5):1162–9. https://doi.org/10.1016/j.fertnstert.2013.01.138.
Donnez J, Dolmans M-M. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–65. https://doi.org/10.1056/NEJMra1614676.
Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;35(4):561–70. https://doi.org/10.1007/s10815-018-1144-2.
Meirow D, Ra’anani H, Shapira M, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–74. https://doi.org/10.1016/j.fertnstert.2016.04.031.
Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031–41. https://doi.org/10.1093/humrep/dew165.
Hourvitz A, Yerushalmi GM, Maman E, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod BioMed Online. 2015;31(4):497–505. https://doi.org/10.1016/j.rbmo.2015.06.025.
Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev. 2017;59:61–70. https://doi.org/10.1016/j.ctrv.2017.07.001.
Lambertini M, Horicks F, Mastro LD, Partridge AH, Demeestere I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev. 2019;72:65–77. https://doi.org/10.1016/j.ctrv.2018.11.006.
Del Mastro L, Ceppi M, Poggio F, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40(5):675–83. https://doi.org/10.1016/j.ctrv.2013.12.001.
Lambertini M, Partridge AH, Del Mastro L. Reply to V. Turan et al. JCO. 2018;37(1):86–8. https://doi.org/10.1200/JCO.18.00630.
Turan V, Bedoschi G, Rodriguez-Wallberg K, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation: lack of biologic basis and the need to prioritize proven methods. JCO. 2018;37(1):84–6. https://doi.org/10.1200/JCO.18.00420.
Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? JCO. 2018;37(6):453–60. https://doi.org/10.1200/JCO.18.01631.
Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. The Lancet Oncology. 2018;19(2):169–80. https://doi.org/10.1016/S1470-2045(17)30891-4.
Stern HJ. Preimplantation genetic diagnosis: prenatal testing for embryos finally achieving its potential. J Clin Med. 2014;3(1):280–309. https://doi.org/10.3390/jcm3010280.
Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, Ethics Committee of the American Society for Reproductive Medicine. Use of preimplantation genetic testing for monogenic defects (PGT-M) for adult-onset conditions: an ethics committee opinion. Fertil Steril. 2018;109(6):989–92. https://doi.org/10.1016/j.fertnstert.2018.04.003.
Daum H, Peretz T, Laufer N. BRCA mutations and reproduction. Fertil Steril. 2018;109(1):33–8. https://doi.org/10.1016/j.fertnstert.2017.12.004.
Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735–42. https://doi.org/10.1200/JCO.2004.05.055.
Quinn GP, Pal T, Murphy D, Vadaparampil ST, Kumar A. High-risk consumers’ perceptions of preimplantation genetic diagnosis for hereditary cancers: a systematic review and meta-analysis. Genet Med. 2012;14(2):191–200. https://doi.org/10.1038/gim.0b013e31822ddc7e.
Mor P, Brennenstuhl S, Metcalfe KA. Uptake of preimplantation genetic diagnosis in female BRCA1 and BRCA2 mutation carriers. J Genet Couns. 2018;27(6):1386–94. https://doi.org/10.1007/s10897-018-0264-2.
Derks-Smeets IAP, Gietel-Habets JJG, Tibben A, et al. Decision-making on preimplantation genetic diagnosis and prenatal diagnosis: a challenge for couples with hereditary breast and ovarian cancer. Hum Reprod. 2014;29(5):1103–12. https://doi.org/10.1093/humrep/deu034.
Acknowledgements
Dr. Sella is a Goldfarb Advanced Fellow in Breast Oncology at DFCI and is also supported by The American Physicians Fellowship for Medicine in Israel and the Pinchas Burstein Talpiot Medical Leadership Program, Chaim Sheba Medical Center, Tel-Hashomer, Israel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tal Sella reports personal fees from Roche outside the submitted work. Ann H. Partridge declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer Genetics
Rights and permissions
About this article
Cite this article
Sella, T., Partridge, A.H. Fertility Counseling and Preservation in Breast Cancer. Curr Breast Cancer Rep 12, 1–12 (2020). https://doi.org/10.1007/s12609-019-00348-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-019-00348-w